Abstract
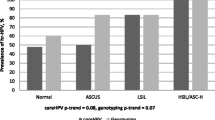
Objective: To compare the performance of human papillomavirus (HPV) assays with conventional Pap cytology for cervical cancer (CC) screening in Mexico. Methods: Pap smears, self-collected vaginal specimens (SS) for HPV testing, and clinician-collected cervical specimens (CS) for HPV testing were obtained from 7868 women, aged 15–85 years old, attending CC screening at the Mexican Institute of Social Security (IMSS) between May and October, 1999. SS and CS specimens were screened for oncogenic HPV DNA by Hybrid Capture 2. Women who received cytological interpretations of atypical squamous cells of undetermined significance (ASCUS), and/or a positive HPV test were referred for colposcopy and histologic studies. The relative estimates for sensitivity, specificity and predictive values of each test were calculated using histological diagnoses of cervical intraepithelial neoplasia (CIN) grades 2 or 3, or CC histological diagnosis. Results: Oncogenic HPV detection rate was 11.6% for SS, and 9.3% for CS. Pap smear abnormalities were observed in 2.4% of the women. Of 1147 women who had at least one abnormal test result, 88.5% underwent colposcopy, and 101 biopsy-confirmed CIN2/3 or cancer cases were identified. The relative sensitivity estimates for the Pap test, SS and CS were 59.4% (95% CI: 49.2–68.9), 71.3% (95% CI: 61.3–79.6), and 93.1% (95% CI: 85.8–96.9), respectively, while the specificities were 98.3% (95% CI: 98.0–98.6), 89.2% (95% CI: 88.5–89.9), and 91.8% (95% CI: 91.2–92.4), respectively. The positive predictive values of Pap, SS and CS were 36.1, 9.1 and 14.9, the colposcopy referrals needed to detect a case of CIN2/3 or cancer were 2.8, 11.0 and 6.7, respectively. Discussion: Both HPV assays detected more cases of CIN2/3 or CC than Pap cytology alone. However, the HPV assays increased the number of colposcopy referrals. Our study suggests that HPV testing could be an effective way to improve the performance of CC screening.
Similar content being viewed by others
References
Lara E, Day NE, Hakama M (1987) Trends in mortality from cervical cancer in the Nordic countries: Association with organized screening programs. Lancet 1: 1247.
Mitchell MF, Tortolero-Luna G, Wright TC, et al. (1996) Cervical human papillomavirus infection and intraepithelial neoplasia: a review. Monogr Natl Cancer Inst 21: 17-25.
World Health Organization (1995) The World Health Report 1995. Bridging the Gaps. Geneva, Switzerland: WHO.
Ponten J, Adami HO, Bergstrom R, et al. (1995) Strategies for global control of cervical cancer. Int J Cancer 60(1): 1-26.
Robles S, White F, Peruga A (1996) Trends in cervical cancer mortality in the Americas. Bull Pan Am Health Org 30: 290.
Beral V, Hermon C, Muñoz N, Devesa S (1994) Cervical cancer. Trends in cancer incidence and mortality. Cancer surveys. Imperial Cancer Res Fund 19/20: 265.
Lazcano EC, Moss S, Alonso P, Salmerón J, Hernández M (1999) Cervical cancer screening in developing countries: why is it ineffective? The case of Mexico. Arch Med Res 30: 240-250.
Hernández M, Lazcano EC, Alonso P, Romieu I (1998) Evaluation of the cervical screening program in Mexico: a population based case control study. Int J Epidemiol 27: 370-378.
Fahey MT, Irwig L, Mascaskill P (1995) Meta-analysis of Pap test accuracy. Am J Epidemiol 141(7): 680-689.
Sasieni PD, Cuzick J, Lynch-Farmery E (1996) NCN Working Group. Estimating the efficacy of screening by auditing smears histories of women with and without cervical cancer. Br J Cancer 73: 1001-1005.
Bonn D, Bradbury J (1998) The warts and all approach to tackling cervical cancer. Lancet 351: 810.
Cuzick J, Meijer CJ, Walboomers JM (1998) Screening for cervical cancer. Lancet 351: 1439-1440.
Scheck A. (1996) Will the HPV DNA test upstage the Pap smear? IVD Technol Mag 8.
Sasieni PD (2000) Human papillomavirus screening and cervical cancer prevention. JAMA 55(4): 216-219.
Cuzick J, Sasieni PD, Davies P, et al. (1999) A systematic review of the role of human papillomavirus testing within a cervical screening programme, Health Technol Assess 3(14): 1-204.
Ratnam S, Franco EL, Ferenczy A (2000) Human papillomavirus testing for primary screening of cervical cancer precursors. Cancer Epidemiol, Biomarkers Prev 9: 945-951.
Schiffman M, Herrero R, Hildesheim A, et al. (2000) HPV DNA testing in cervical cancer screening: results from women in a high-risk province of Costa Rica. JAMA 283(1): 87-93.
Cuzick J, Beverly E, Ho L, et al. (1999) HPV testing in primary screening of older women. Br J Cancer 81(3): 554-558.
Clavel C, Masure M, Bory JP, et al. (1999) Hybrid Capture II-base human papillomavirus detection, a sensitive test to detect in routine high-grade cervical lesions: a primary study on 1518 women. Br J Cancer 80(9): 1306-1311.
Wright TC, Denny L, Kuhn L, Polleck A, Lorincz A (2000) HPV-DNA testing of self-collected vaginal samples compared with cytologic screening to detect cervical cancer. JAMA 283: 81-86.
Flores Y, Shah KV, Lazcano EC, et al. (2002) Design and methods of the evaluation of an HPV-based cervical cancer screening strategy in Mexico: the Morelos HPV Study. Salud Pública de México 44(4): 335-344.
Manual of the Histologic Staining Methods of the Armed Forces (1968) Institute of Pathology, 3rd edn, p. 68.
Luff RD (1992) The Bethesda System for reporting cervical/vagina cytologic diagnoses: report of the 1991 Bethesda workshop. Hum Pathol 23: 719-721.
Lorincz A (1996) Hybrid CaptureTM method for detection of human papillomavirus DNA in clinical specimens. Papillomavirus Report 7: 1-5.
Reid R (1990) Colposcopy of cervical preinvasive neoplasia. In: Singer A, ed. Premalignant Lesions of the Lower Genital Tract. Vol. 2. New York: Elsevier, 87-116.
Meijier CJ, Rozendaal L, van der Linden JC, et al. (1997) Human papillomavirus testing for primary cervical cancer screening. In: Franco E, Monsonegro J, eds. New Developments in Cervical Screening and Prevention. Oxford Blackwell Science, 338-357.
Cuzick J, Sasieni P (1997) Estimates of the cost impact to introducing HPV testing into a cervical screening programme. In: Franco E, Monsonegro J, eds. New Developments in Cervical Screening and Prevention. Oxford Blackwell Science, 364-372.
Lazcano-Ponce E, Alonso P, López L (1997) Validity and reproducibility of cytologic diagnosis in a sample of cervical cancer screening centers in México. Acta Cytológica 41(29): 277-284.
Sellers JW, Lorincz AT, Mohany JB, et al. (2000) Comparison of self-collected vaginal, vulvar and urine samples with physicianscollected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ 163(5): 513-518.
Kenney W, Sung HY, Kearney KA, Miller A, Sawaya G, Hiatt PA (1998) Missed opportunities for cervical cancer screening of HMO members developing invasive cervical cancer. Gynecol Oncol 71: 428-430.
Dzuba IG, Allen B, Flores Y, et al. (2002) The acceptability of selfcollected samples for HPV testing vs. the Pap test as alternatives in cervical cancer screening. J Women´s Health Gender-Based Med 11(3): 265-275.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salmerón, J., Lazcano-Ponce, E., Lorincz, A. et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 14, 505–512 (2003). https://doi.org/10.1023/A:1024806707399
Issue Date:
DOI: https://doi.org/10.1023/A:1024806707399