-
PDF
- Split View
-
Views
-
Cite
Cite
Vicente Falcó, Dolors Rodríguez, Esteban Ribera, Esteban Martínez, José Maria Miró, Pere Domingo, Ruth Diazaraque, R. Arribas José, Juan J. González-García, Francesc Montero, Lluis Sánchez, Albert Pahissa, Severe Nucleoside-Associated Lactic Acidosis in Human Immunodeficiency Virus–Infected Patients: Report of 12 Cases and Review of the Literature, Clinical Infectious Diseases, Volume 34, Issue 6, 15 March 2002, Pages 838–846, https://doi.org/10.1086/339041
Close - Share Icon Share
Abstract
Lactic acidosis is a rare but often fatal complication reported in some human immunodeficiency virus (HIV)–infected patients treated with nucleoside-analogue reverse-transcriptase inhibitors. We report a series of 12 patients with HIV infection treated with nucleoside analogues who developed unexplained metabolic acidosis. We have also reviewed 60 additional published cases. The aim of the present study is to describe the clinical picture, prognostic factors, and final outcome for nucleoside-associated lactic acidosis. The mortality rate is high: 33% for our patients, and 57% for the patients described in the literature. In the multivariate analysis, a lactate serum level of >10 mM (odds ratio [OR], 13.23; 95% confidence interval [CI], 2.96–59.25) was the only factor associated with higher mortality. The administration of specific therapy with cofactors against acidosis was associated with a lower mortality (OR, 0.17; 95% CI, 0.04–0.73). We conclude that specific therapy with cofactors may improve the outcome for patients with this syndrome.
The prognosis of patients with HIV infection has improved dramatically since the introduction of highly active antiretroviral therapy (HAART). However, the increase in life expectancy and the need for permanent antiretroviral therapy has led to the description of new and sometimes serious adverse effects.
In 1991, Lai et al. [1] described a patient with fulminant hepatic failure associated with the use of didanosine. This patient also had severe lactic acidosis and died a few days after presentation. Autopsy revealed diffuse microvesicular hepatic steatosis. Since then, this rare but often life-threatening syndrome, now named “severe nucleoside-associated lactic acidosis” (NALA) has been reported increasingly often [2–9]. Hepatic steatosis and lactic acidosis are thought to be caused by nucleoside reverse-transcriptase inhibitor (NRTI)–associated mitochondrial toxicity that results in inhibition of mitochondrial DNA γ-polymerase, which, in turn, impairs synthesis of mitochondrial enzymes that generate adenosine triphosphate [10].
Low levels of hyperlactatemia have been reported in 21% of NRTI-treated patients [11], although the majority of these patients are asymptomatic. Symptomatic hyperlactatemia is less common; the incidence varies from 1.7 to 25.2 cases per 1000 person-years of treatment [12, 13]. This high variation in the calculated incidence is the result of the variety of case definitions used. Only a minority of patients develop the most severe form of hyperlactatemia, which presents as metabolic acidosis and is associated with a high mortality rate. At present, the exact incidence of the most severe form of hyperlactatemia is unknown. Nor is it known which asymptomatic patients with elevated serum lactate levels have an increased risk for developing metabolic acidosis.
Most of the cases of severe NALA that have been described are single case reports or series of cases with a small number of patients. To our knowledge, a comprehensive review of all reported cases of severe NALA has not been published in the English-language literature. We describe 12 HIV-infected patients who received treatment with different antiretroviral regimens and who developed severe NALA. Additionally, we have performed an extensive literature review of the published reports of cases of NALA. The aim of the present study is to describe the clinical picture, prognostic factors, and final outcome for patients with NALA.
Patients and Methods
From 1997 through 2000, we identified 12 HIV-infected patients treated with NRTIs who developed unexplained metabolic acidosis. Patients were treated in 4 hospitals in Spain: Hospital Universitari Vall d'Hebron, Hospital Clínic Universitari, and Hospital de la Santa Creu i Sant Pau (Barcelona), and Hospital Universitario La Paz (Madrid). A total of 5400 patients (14,040 person-years) treated with NRTIs were observed in these hospitals during the study period. In all cases, known causes of lactic acidosis other than antiretroviral therapy were ruled out. For each patient, we recorded the following data: age; sex; years since diagnosis of HIV infection; antiretroviral drugs received; duration of treatment with NRTIs; prior opportunistic infections; clinical manifestations; CD4 lymphocyte count; plasma HIV RNA load; underlying hepatic disease; serum values for pH, value, bicarbonate, base excess, anion gap, lactate, aspartate transaminase, alanine transaminase, and creatine; and therapy received and outcome. Lactate, pH, bicarbonate, and base excess levels were measured in venous blood samples.
The literature review was performed with use of the MEDLINE, AIDSLINE, Healthgate, and PubMed databases to search for reports of severe NALA. We selected for review all cases that fulfilled the following criteria: (1) the patient was an adult with HIV infection who was treated with antiretroviral drugs, (2) the patient had confirmed metabolic acidosis (low pH levels and/or low bicarbonate levels), and (3) other known causes of lactic acidosis, such as infection, hypoxemia, exogenous intoxication, shock, or malignancy, had been excluded. Cases were excluded from the review if (1) the patient had high serum lactate levels but no acidosis or (2) the patient had hepatic steatosis and/or pancreatitis but metabolic acidosis was not documented or was not indicated in the report. From 2 reports [4, 14], we selected only the cases that fulfilled the established inclusion criteria. We recorded the following available data for the patients from each report: age; sex; antiretroviral drugs used; duration of exposure to NRTIs; CD4 lymphocyte count; plasma HIV RNA load; underlying hepatic disease; serum values of pH, bicarbonate, lactate, AST and ALT; and therapy received and outcome. Specific therapy for lactic acidosis was defined as administration of ⩾1 of the following cofactors: thiamine, riboflavin, L-carnitine, coenzyme Q, and prostaglandin E.
We included in the statistical analysis the 60 patients from the literature review and the 12 additional patients described in this article. All statistical analyses were performed with the SPSS software package (version 10.0). The χ2 test or Fisher's exact test was used to assess the association between categorical variables (treatment with zidovudine, stavudine, didanosine, or lamivudine and administration of specific therapy with cofactors) and mortality. The association of continuous variables with mortality was analyzed by means of Student's t test.
We used a multiple logistic regression model stepwise forward to assess the association between mortality and serum lactate levels, type of antiretroviral drug used, and administration of specific therapy for lactic acidosis with cofactors. These variables were chosen because they had been significantly associated with mortality in the univariate analysis. The cutoff point that better differentiated between both categories of the dependent variable in the logistic regression model was arbitrarily chosen for the continuous variables. Adjusted ORs and 95% CIs were calculated. Logistic regression analysis was performed for only 56 patients. Sixteen cases were excluded because some data were lacking. Statistical significance was defined as P < .05.
Results
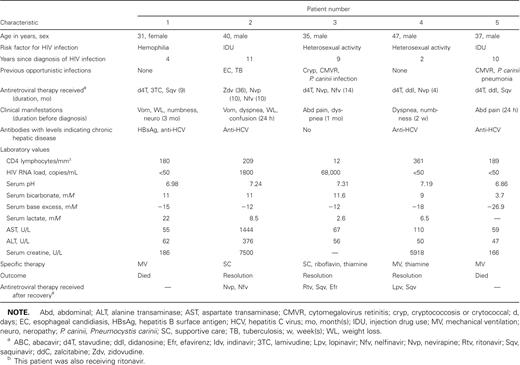
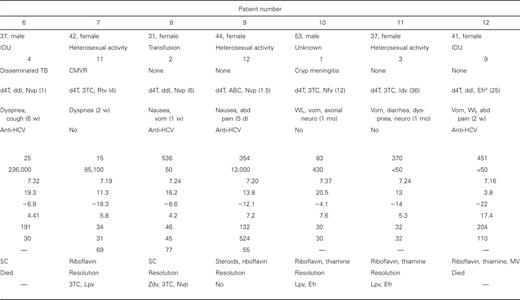
Acute metabolic acidosis was diagnosed in 12 HIV-infected patients who received nucleoside analogues, which represents an estimated incidence of 0.85 cases per 1000 patient-years of antiretroviral therapy. The characteristics of the patients are summarized in table 1. Six patients were men, and 6 were women, with a median age of 38.5 years (range, 31–53 years). Time from diagnosis of HIV infection to onset of symptoms ranged from 1 to 12 years. Median CD4 cell count was 199 lymphocytes/mm3 (range, 12–536 lymphocytes/mm3), and 6 patients (50%) had >200 CD4 lymphocytes/mm3. Plasma HIV RNA ranged from <50 copies/mL (in 6 patients) to 236,000 copies/mL.
Summary of clinical characteristics of HIV-infected patients with metabolic acidosis secondary to nucleoside reverse-transcriptase inhibitor (NRTI) therapy.
All patients had been receiving HAART that contained 1 or 2 NRTIs for 1–36 months before the onset of symptoms. Zidovudine was the implicated drug in 1 case. Eleven patients received stavudine alone or in association with other NRTIs (stavudine, 1 patient; stavudine and didanosine, 5; stavudine and lamivudine, 4; and stavudine and abacavir, 1).
Clinical manifestations were gastrointestinal symptoms (10 patients), cough and dyspnea (6), weight loss (4), numbness (2), and painful dysesthesias (2). Three patients received a diagnosis of severe axonal neuropathy of subacute onset in addition to metabolic acidosis. Two patients presented with acute symptoms (⩽24 h after onset). The remaining 10 patients had manifestations for 1 week to 3 months before the diagnosis was made. All patients had evidence of metabolic acidosis, with low pH (median, 7.22; range, 6.86–7.37) and/or bicarbonate levels (median, 11.45 mM; range, 3.7–20.5 mM). Data on serum lactate levels were available for 11 patients (median, 6.5 mM; range, 2.6–22 mM) . Only 2 patients showed a significant increase (to 5 times greater than the normal value) in transaminase levels.
On diagnosis of metabolic acidosis, antiretroviral therapy was discontinued for all patients. Treatment consisted of supportive measures (administration of fluids and bicarbonate). Five patients required admission to the intensive care unit. Therapy with cofactors, such as thiamine and/or riboflavin, was administered to 7 patients for 10–21 days. Four (33.3%) of our 12 patients died. Necropsy was performed for only 1 patient, and the major finding was hepatic steatosis. For the 8 patients who survived, clinical manifestations disappeared 1–24 weeks after discontinuation of antiretroviral therapy. For the 2 patients with severe axonal neuropathy who survived, neuropathic symptoms resolved very slowly (up to 1 year after discontinuation of nucleoside analogue therapy). After recovery, antiretroviral therapy was reinstituted for 7 patients (table 1). For 5 of these 7 patients, an NRTI-sparing regimen was chosen, and 2 patients were treated with new NRTI regimens, both of which included lamivudine and one of which included zidovudine.
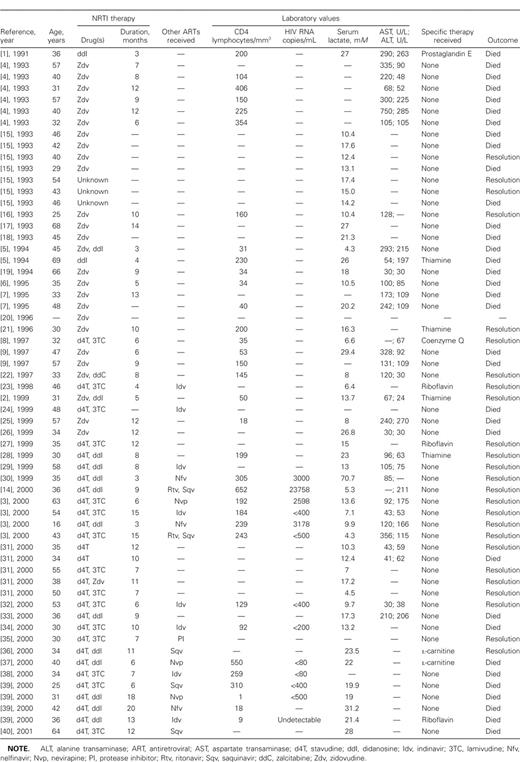
Literature review. We reviewed 60 cases of severe NALA described in the literature (table 2). The CD4 T cell count ranged from 1 to 652 lymphocytes/mm3 (median, 155 lymphocytes/mm3) in the 34 patients for whom this parameter was reported. In 13 (38.2%) of these 34 patients, the CD4 count was ⩾200 lymphocytes/mm3. Data on plasma HIV RNA levels was available for only 13 patients; levels ranged from below detection (<50 copies/mL) to 23,758 copies/mL. Nine (69.2%) of these 13 patients had plasma HIV RNA levels of <50 copies/mL. The most frequent symptoms at presentation were nausea (40 of 51 patients for whom this parameter was recorded), abdominal pain (39 of 50), vomiting (36 of 46), dyspnea (29 of 37), weight loss (22 of 28), and paresthesias (12 of 15). Body mass index was reported for 21 patients and varied from 20.1 to 46.
All patients were receiving NRTIs, as follows: stavudine, 29 (48.3%) of patients; zidovudine, 27 (45%); didanosine, 15 (25%); lamivudine, 15 (25%); and zalcitabine, 1 (2%); for 3 patients, the NRTI received was not reported. Twenty-seven patients were receiving only 1 NRTI, and 30 were receiving combinations of NRTIs. The most frequent combinations were stavudine and lamivudine (15 patients) and stavudine and didanosine (11 patients). Twenty of 60 patients received HAART; 17 of these 20 received a protease inhibitor, and 3 received nevirapine in association with NRTIs. Patients had been treated with antiretroviral therapy for 3–20 months (median, 9 months) before the onset of symptoms. Lactate levels were noted for 48 patients and were elevated in all cases (median, 14.6 mM; range, 4.3–70.7 mM).
Liver biopsies were performed in 34 cases. Findings were usually described as microvesicular or macrovesicular steatosis. Muscle biopsies were performed in 8 cases and showed red-ragged fibers in 5 cases, lipid droplets in another 5, muscle fiber atrophy in 1, abnormal mitochondrial architecture in 1, decreased mitochondrial DNA content in 1, and normal findings in 1.
All patients were treated with supportive care, including intensive care unit measures when required and high-dose bicarbonate intravenous infusion. Five patients underwent hemodialysis. Eleven patients received therapy with cofactors, as follows: thiamine, 4 patients; riboflavin, 3; L-carnitine, 2; prostaglandin E, 1; and coenzyme Q, 1. For 1 patient, in whom zidovudine had been implicated in NALA, zidovudine was reinstituted, and lactic acidosis recurred 14 months later. Four other patients who were receiving stavudine when lactic acidosis developed were switched to another NRTI when they recovered, and there were no recurrences. It is important to note that 3 of these 4 patients were receiving lamivudine when lactic acidosis developed, and the condition did not recur when lamivudine was reinstituted.
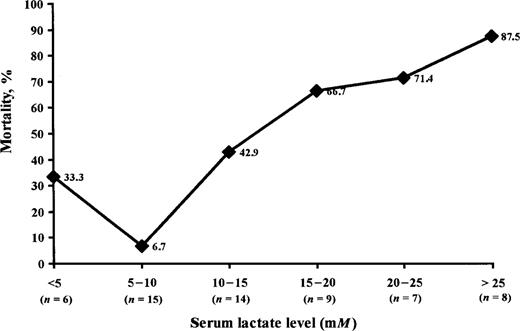
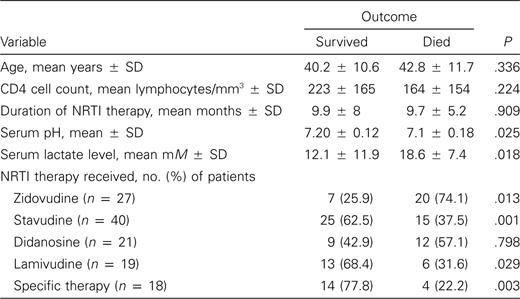
Analysis of mortality. Thirty-four patients (56.6%) died as a consequence of NALA syndrome. In the univariate analysis, serum lactate level, serum pH, and use of zidovudine were associated with higher mortality rates. On the other hand, use of stavudine, use of lamivudine, and administration of specific therapy with cofactors against lactic acidosis were associated with lower mortality rates (table 3). Figure 1 shows the relationship between serum lactate levels and mortality.
Case summaries for HIV-infected patients with metabolic acidosis secondary to nucleoside reverse-transcriptase inhibitor (NRTI) therapy who have been described in the literature.
Relationship between serum lactate levels and mortality for HIV-infected patients with metabolic lactic acidosis secondary to nucleoside reverse-transcriptase therapy.
When the multivariate analysis was performed, only a serum lactate level of >10 mM was associated with higher mortality (OR, 13.23; 95% CI, 2.96–59.25; P = .001). In contrast, the administration of therapy with cofactors against acidosis was significantly associated with lower mortality (OR, 0.17; 95% CI, 0.04–0.73; P = .017). Use of specific nucleoside analogues was no longer significant in the multivariate model. The administration of specific therapy was directly associated with the use of stavudine (P = .001) and lamivudine (P = .353) and inversely associated with the use of zidovudine (P = .001).
Discussion
Nucleoside analogues inhibit mitochondrial DNA polymerase–γ. This inhibition leads to a depletion of mitochondrial DNA and a subsequent alteration in the synthesis of mitochondrial proteins [41, 42]. Most of the adverse effects attributed to the NRTIs might be explained by mitochondrial toxicity. Although all NRTIs have been implicated in cases of NALA, most cases have been associated with stavudine therapy. This observation is in agreement with the relative potencies of various nucleoside analogues with regard to their inhibitory effect on mitochondrial DNA production in vitro; the relative potencies, from most potent to least, are as follows: zalcitabine ⩾ didanosine ⩾ stavudine> lamivudine> zidovudine> abacavir [42]. Abacavir and tenofovir have a very low affinity for mitochondrial DNA polymerase–γ [43].
Cases of NALA have been reported as soon as 1 month and as late as 20 months after the start of antiretroviral treatment. The reasons that only a minority of patients who receive therapy with NRTIs develop mitochondrial toxicity are still unknown. Of note, HIV-infected women appear to be overrepresented both in our series of cases (50%) and in the cases described in the literature (42%). This percentage is substantially higher than the percentage of HIV-infected women in the developed world (20%) [44], which suggests that HIV-infected women might have a higher risk of developing severe NALA. It is also interesting that, in the cases in which the patient's weight was reported, patients with NALA had, in general, a high body mass index. Finally, it has been argued that deficiencies in riboflavin and thiamine, cofactors required for oxidative phosphorylation, may predispose patients to the development of lactic acidosis [23].
Another important observation is that NALA can develop at any stage of HIV disease. Six of our patients developed this complication when their CD4 lymphocyte count was >200 lymphocytes/mm3, and 6 developed it when their virus load was undetectable.
It seems that hyperlactatemia can produce a disease with a wide spectrum of manifestations, including asymptomatic patients [14, 45, 46], patients with mild symptoms [34], patients in whom hyperlactatemia is associated with lipodystrophy [47], and patients (included in our review) who develop severe NALA. In our series of patients, the main clinical manifestations were dyspnea, abdominal pain, vomiting, and weight loss. Three of our patients suffered severe axonal neuropathy along with NALA. To our knowledge, severe neuropathy associated with NALA has been reported only once before [48]. Interestingly, a recent study has shown the utility of serum lactic acid levels in distinguishing between HIV- and nucleoside-associated axonal neuropathy [49].
Treatment of NALA is mainly supportive, consisting of fluid and bicarbonate administration and respiratory support when needed. It seems essential to discontinue all antiretroviral drugs; however, surviving patients often need to continue antiretroviral therapy. Nonnucleoside reverse-transcriptase inhibitors (NNRTIs) lack affinity for mitochondrial DNA polymerases [10], so it has been suggested that combinations of NNRTIs and protease inhibitors could be a safe treatment regimen for these patients [32]. Although some patients have tolerated rechallenge with a new NRTI-containing regimen, we believe that there are insufficient data to recommend this strategy at present and that treatment with an NRTI-sparing regimen is probably the best option. However, for patients for whom it is difficult to find a satisfactory regimen of antiretroviral drugs because of problems of resistance to NNRTIs or protease inhibitors, careful switching to another NRTI after recovery may be justified. Four patients who developed NALA while receiving stavudine were switched to another NRTI after recovery, without recurrence of NALA [3, 8]. It seems likely that lamivudine can be safely administered to such patients. In fact, the 4 patients mentioned above were receiving treatment with lamivudine and stavudine when lactic acidosis developed, and, after recovery from NALA, they were rechallenged with lamivudine in association with an NRTI other than stavudine [3, 8]. Two of our patients were treated, after recovery from NALA, with a regimen that included NRTIs. In 1 case, stavudine was switched to zidovudine, and, in both cases, lamivudine was added. Neither of these patients had a recurrence of lactic acidosis.
In a recent longitudinal study of venous lactate concentrations in patients receiving antiretroviral therapy, John et al. [50] described 5 patients who presented with moderate hyperlactatemia without acidosis while receiving treatment with stavudine and who, when treatment was changed to zidovudine or abacavir, experienced relief of their symptoms. Treatment with abacavir and tenofovir, because of their low affinity for mitochondrial DNA polymerase–γ, also might be an alternative [43, 44]. In any case, serum lactate levels should be closely monitored in patients rechallenged with an NRTI or tenofovir, and interruption of therapy is mandatory if serum lactate levels increase.
Administration of essential cofactors such as thiamine, riboflavin, L-carnitine, vitamin C, and antioxidants have been used as therapy for congenital mitochondrial diseases [41]. Although no studies have proved the beneficial effects of these substances for patients with NALA, a few published case reports have suggested that they may induce recovery from lactic acidosis [23, 27, 51]. Among our 12 patients, 7 were treated with riboflavin and/or thiamine, and only 1 died. The literature describes 11 patients who received specific therapies with essential cofactors. Only 3 of them died, which corresponds to a lower mortality rate than the global mortality rate found for all the patients whose cases we reviewed. In the multivariate analysis, the only variable that was significantly associated with a lower mortality rate among patients with NALA was the administration of specific therapy with essential cofactors. Because the incidence of this severe complication is extremely low, a randomized trial to assess the usefulness of such therapy is not feasible at present. Our analysis is retrospective and might be biased by the underreporting of cases of NALA that were fatal despite the use of essential cofactors. However, given the poor prognosis for patients with NALA and the lack of other proven beneficial interventions, we favor the use of essential cofactors in the treatment of HIV-infected patients with NALA.
The mortality rate among patients with lactic acidosis is very high: 33% for our series of patients and 57% for the patients described in the literature. In our analysis of the variables associated with mortality, we found that the age of the patients, CD4 lymphocyte count, and treatment with NRTIs were not associated with mortality. The only variable that was independently associated with mortality was a serum lactate level >10 mM. We found a strongly positive correlation between mortality and having a serum lactate level of >10 mM.
Several issues concerning NALA remain unsolved, especially with regard to prevention and treatment. Although the efficacy of the essential cofactors remains to be determined, our study suggests that they may be useful. Moreover, treatment with these agents does not imply a risk of toxicity or intolerance. Therefore, we recommend their use in patients with this rare and severe complication of NRTI therapy.
acknowledgments
We thank Celine L. Cavallo for her assistance with English corrections.
references
Figures and Tables
Variables associated with death, according to univariate analysis, for patients receiving nucleoside reverse-transcriptase inhibitor (NRTI) therapy.





Comments