Article Text
Abstract
Objectives: To describe recent trends in age at first sex in African countries, identifying and making due allowances for a variety of common reporting errors.
Methods: Demographic and Health Surveys (DHS) data from six African countries conducting three or more surveys since 1985 were analysed using survival analysis techniques, combining information on virginity status and retrospective reporting of age at first sex. Hazard analysis was used to separate the effects of reporting error and compositional change and to estimate true changes in sexual debut over time. A multistate life table analysis incorporating transition to first marriage allowed cohorts to be classified according to person years spent as virgins, as sexually active unmarried, and married.
Results: Intersurvey comparisons generally suggested a slow secular rise in age at first sex. However, tracing birth cohorts between surveys revealed inconsistencies—median ages reported by female members of a birth cohort in their teens were generally higher than those reported when they reached their twenties, even when allowing for residence and education changes—probably a result of young, sexually active women denying they had ever had sex. Male birth cohorts tend to display the opposite kind of bias.
Conclusions: Uganda, Kenya, and Ghana have experienced a more pronounced and unambiguous decline in premarital sexual activity than Tanzania, Zambia, and Zimbabwe, with statistically significant increases in age at first sex. In addition, Uganda has maintained a very short interval between onset of sexual activity and marriage for both sexes.
- DHS, Demographic and Health Surveys
- sexual debut
- premarital sex
- survival analysis
- reporting bias
Statistics from Altmetric.com
Age at first sex is an important indicator of exposure to risk of pregnancy and sexually transmitted infections during adolescence. In fertility studies age at first marriage is often used as a proxy measure of the onset of a woman’s exposure to pregnancy, but in many societies premarital sexual activity is common, and age at first sex would be a better proxy.1 In the context of the AIDS pandemic, accurate monitoring of trends in age at first sex has become increasingly important, as interventions target youth and discourage premarital sexual activity. In several countries HIV prevalence among pregnant women attending antenatal clinics has declined in younger age groups, but not among older women.2,3 Such changes may be associated with changes in age at first sex, rates of partner change, sexual mixing patterns, and condom use. In Uganda, a rapid increase in age at first sex in urban areas between 1990 and 1995 was considered a major contributing factor in the observed HIV prevalence decline in young pregnant women from about 1993.4
Various indicators have been used to measure age at first sex from cross sectional data in a single survey and to assess change over time from data in multiple surveys,4,5,6,7,8,9,10 but there are many examples of flawed statistical constructs and inefficient use of data. Common problems include failure to allow for age censoring,6–8 comparison of crude proportions without allowing for age structure,9 and failure to combine current status and recall data to minimise sampling error.5,10
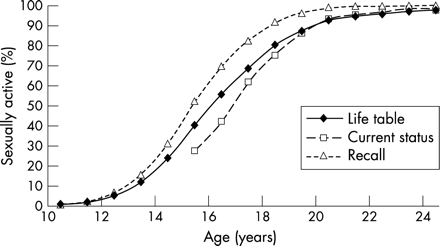
These patterns are illustrated in figure 1 based on Kenyan DHS data for 1993,11 which compares the cumulative onset of first sex obtained using the life table method with estimates obtained by looking only at current status data and only at recall data for those who have ever had sex. It clearly shows that recall data alone would produce a younger distribution than estimates based on the full data set, including those who never had sex. This difference is inevitable—whatever the empirical data source used. However, theoretical considerations alone would not necessarily lead us to expect a systematic difference between the current status curve and the life table curve, unless there was an underlying trend in age of sexual debut over time, or if the data were affected by reporting errors. This paper explains how data from successive surveys can be used to discriminate between these two possibilities, and how life table techniques can be adapted to describe and explain trends in age at first sex and age at first marriage.
Example of different methods to assess age at first sex: Kenya DHS 1993, women aged 15–24 years, proportion sexually active by age.
The techniques are applied to analyse changes in age at first sex in six African countries that conducted three or more DHS since 1990. Uganda is one of these countries, and it has been widely reported that a decline in premarital sexual activity in that country has been a major factor in the observed decline in HIV prevalence in the late 1990s.4 It is therefore of particular interest to evaluate the evidence on trends in age at first sex in Uganda, and to compare the scale of the change—and its robustness in the face of reporting errors—with similar data from other African countries.
METHODS AND DATA
The most appropriate method for estimating the distribution of age at first sex from censored observations is survival analysis.12 Maximum likelihood methods could be used, but these are more cumbersome, and require assumptions about the underlying parametric form of the distribution.13 The input data for survival analyses are age of the respondent, whether or not they ever had sex and, if applicable, recalled age at first sex. In calculating a survival function describing the probability of remaining a virgin, reported age at first sex is the failure event and those who never had intercourse are censored at their current age. Most standard statistical analysis packages provide routines for generating and comparing life tables based on person years of exposure at each single year of age and output the cumulative risk for becoming sexually active, allowing for censoring effects.
For 15–19 year olds this approach leads to large values for person years exposed and thus smaller sampling errors than relying on current status data alone. For 20–24 year olds the difference in estimates obtained by the life table method and those obtained using only recall data is minor if the median age at first sex is below 20 years of age. However, even in older groups it can yield more robust estimates if the analysis includes subgroups with a relatively late sexual debut. Life table construction based on data linked over a wide range of ages implies an assumption about homogeneity of behaviour across the age range, which may not be appropriate in times of rapid change. Five year age groups should generally be used for this type of analysis; life tables for 10 year age groups may mask changes between adjacent cohorts.
Several measures can be used to summarise the distribution of age at first sex. The median and interquartile ranges are simple indices that capture the location and shape of the survival curve. If median age at first sex is beyond the upper age limit of the age group (for example, data for teenage respondents collected in a school survey), a model can be fitted to the observed distribution of survival time to predict median age at first sex. The gamma distribution has been found to provide a good fit for the initiation of sexual activity.14
Linear rank statistical tests, such as the Wilcoxon rank test and the log rank test, can be used to assess equivalence in survival functions.12 The log rank test gives more weight to later events than the Wilcoxon rank test, and is more appropriate if divergence of the survival curves occurs principally at older ages, and does not require the assumption that initiation of first sex corresponds to a constant age specific force. However, for weighted data, a Cox regression based test for equality of survival curves15 is more appropriate.
The data sources used for this illustrative analysis were drawn from Demographic and Health Surveys (DHS) conducted between 1988 and 2001 in six African countries: Ghana, Kenya, Tanzania, Uganda, Zambia, and Zimbabwe, selected because each had three surveys in this time period.11,16–32 Table 1 gives survey dates, and sizes of the male and female populations aged under 25, which form the basis of most of the subsequent tables. Not all the surveys included men, and samples of men were 20–80% smaller than those of women. Wherever possible, parallel analyses are performed for males and females, but inevitably the scope, strength, and significance of the results for males are lower than for females. Most analyses in this paper compare observations of the 15–24 years age group, in some cases splitting it into teenagers (aged 15–19 years) and 20–24 year olds. For some analyses, it is useful to examine birth cohorts as they appear in different surveys. The cohorts identified for comparison correspond to those aged 15–19 years (youngest), 20–24 years (middle), and 25–29 years (oldest) at the last survey, with birth year used to identify the same cohort at different time points.
DHS survey years, birth cohorts, and population size used for analysis
RESULTS
Table 2 shows how median age at first sex and the interquartile range have changed over the series of three surveys in each of the six countries for the 15–24 year age group. The median age at first sex is a general measure of the youthfulness of the start of sexual activity, the interquartile range (number of years between the age at which 25% have experienced sexual activity and the age at which 75% are sexually active) tells us how quickly sexual activity builds up among the youthful population once it has been initiated by a substantial minority. Whenever two surveys are compared, differences that are statistically significant are indicated by presenting the results of the latter survey in bold type.
Median age at first sex (with interquartile range), by sex, country and survey, for respondents aged 15–24 years at survey
There is a wide diversity of patterns, trends, and sex differentials in age at first sex in these six countries. Median age ranges from 16.2–18.6 years for females, 15.8–18.6 years for males; the interquartile range varies from 2.9–4.5 years for females, 3.3–5.2 years for males. Sex differentials are not uniform, with males having an older age at first sex and slower progression than females in Ghana and Uganda, with the reverse for Kenya. Historically, Uganda recorded the lowest female age at first sex, but in recent times Zambian women reported ages equally as low. Females in Zimbabwe report the oldest starting ages for sexual activity, and the slowest progression.
One problem with the analysis in table 2 is that inclusion of respondents over 20 years makes the picture less current. Recall data among older informants (20–24 year olds) provide information on age at first sex a number of years before the survey. If most 20–24 year olds report that their age at first sex was 17, then their first sexual experience occurred on average 3–7 years before the time of the survey. More recent data on trends are often required for programme planning and evaluation purposes, especially in the context of AIDS epidemics. Furthermore, if the surveys are less than 10 years apart, the overlap in age cohorts will obscure true trends among teenagers. For this reason it is useful to look separately at trends based on respondents aged less than 20 years (table 3).
Median age at first sex, by sex, age group, country, and survey
An examination of differences between age groups within each survey shows that in Kenya and Uganda 15–19 year old females report a later age at first sex than those aged 20–24 years at each survey, suggesting a rise in age at sexual debut over time. For Tanzanian females the opposite is true—these implied trends are consistent with the between survey changes noted in table 2. The magnitude of differences between age groups tends to be larger than the between survey changes noted previously: differences of more than half a year in median age are seen for females in Kenya and Zimbabwe. In Kenya and Uganda the between age group differences attain statistical significance at each survey.
As surveys are generally 4–6 years apart, the 15–19 year age group in an earlier survey should correspond approximately to the 20–24 year age group in the following one. Unlike the mean, which is influenced by later events, median age at first sex should not change as a cohort ages from one survey to the next. However, many contradictions are apparent, with some female cohorts in Ghana and Zimbabwe and male cohorts in Ghana, Tanzania, and Zambia showing unexpectedly large variation in median age at first sex between surveys. To examine this problem more closely, in table 4 cohorts are classified by exact year of birth rather than age at survey. The cohort birth years are shown in table 1.
Median age at first sex by country, cohort, sex, and age at survey (pooled data)
Overall trends between birth cohorts replicate patterns seen in table 2; however, where a cohort is captured at different reporting ages in successive surveys, various inconsistencies become apparent. In general, male cohorts report an older age at first sex when questioned at later ages, whereas females tend to report younger age. The exceptions to this general observation are Kenyan men and Ugandan women who gave consistent reports at all ages, and Zimbabwean men and Ghanian women, who showed a reporting bias in the opposite direction to that which is typical for their sex. Figure 2 illustrates the magnitude of these errors in Tanzanian males and Kenyan females.
Within-cohort inconsistency in median age at first sex: (A) Tanzanian males, (B) Kenyan females.
The fact that different patterns are observed for different countries suggests that a variety of reporting errors may occur with different prominence. If early sexual experience is misreported in the light of campaigns promoting abstinence for youth we would expect two types of reporting error: (1) young, sexually active respondents may deny having ever had sex, and (2) among older respondents (many of whom would be married and therefore not need to deny sexual activity) there may be a tendency to report that first sex had occurred at a later age than it had—particularly if first sexual encounter was with someone other than their spouse. The first of these two errors would produce the reporting pattern identified above as typical of females, with falling age at first sex reported as the cohort aged; the second would produce the opposite pattern, more often seen in male cohorts in these data sets.
A different type of reporting error may occur in spite of campaigns promoting sexual abstinence: if teenage virgins felt the need to claim they were sexually experienced this would lower the median age at first sex for the cohort at young reporting ages, and could also partly explain the typical reporting pattern for males. If HIV related mortality starts to deplete cohorts before they reach their mid-twenties, we would observe a real increase in median age at first sex as cohorts aged, because those who became sexually active earliest would also face the risk of HIV infection earlier, and thus a greater chance of premature AIDS related death.
Within-cohort inconsistencies could also be a result of selection effects if samples drawn in consecutive surveys were not nationally representative at all ages. For example, if age at first sex is strongly correlated with education, and one survey happens to under- or overrepresent respondents with secondary education, a spurious difference by survey (and thus by reporting age) would be produced within-birth cohorts observed in that survey. Note that real changes over time in national education levels would not produce the within-birth cohort discrepancies shown above; the changes over time may be partly real, and partly a result of an imperfect sampling strategy.
Secondary education and urban residence are associated with later age at first sex for both males and females. Differences in median age at first sex between those with secondary education and those with primary schooling are between 1.5–2 years for females, 0.5 years for males, and are more consistent and statistically stronger than the intersurvey, interage group, and intercohort differences discussed earlier.33 Declines in the proportion of males with secondary education between the earlier and later surveys are observed in all six countries, alongside increases in the urban proportions for both sexes. Rural to urban migration flows and changes in high school enrolment should not cause discrepancies in reporting age at first sex within-birth cohorts, unless there was a tendency to under sample the mobile population or those continuing in higher education in some surveys.
We can asses the scale of the bias due to reporting at different ages, as well as the effects of changes in education and residence, both real (due to changes in composition of the population over time) and spurious (due to imperfect sampling), on the reported median age at first sex, by constructing virginity life tables for birth cohorts adjusted for education, residence, and reporting age. The results of this procedure are shown in table 5. The top panel for each sex shows the difference in median age at first sex for successive cohorts using pooled data from all available surveys, the middle panel shows the same differences after adjustment for the effects of education and residence, and the bottom panel includes an additional adjustment for age at survey. Positive differences imply a trend over time towards older age at first sex.
Unadjusted and adjusted cohort differences in median age at first sex, allowing for changes in reporting age, education, and residence
For males, adjustment generally results in a loss of significance in the apparent cohort trends, except for Uganda and Tanzania, where there is a larger and more significant increase in age at first sex between the middle and oldest cohorts after adjustment. In the case of females, trends in Ghana, Kenya, and Uganda retain their significance after adjustment, the main increase occurring between the middle and youngest cohorts. The Tanzanian female intercohort differences lose significance after adjusting for reporting age.
Simply initiating sex does not put someone at risk of HIV—infection risks arise if sex occurs with partners who currently have, or have had other partners. An emphasis on current partnerships and a widespread perception that spouses are more likely to be monogamous than premarital or extramarital partners, engenders the belief that being married carries a lower risk of HIV infection than engaging in premarital sex. However, coital frequency within marriage tends to be higher than in non-marital partnerships, and condom use tends to be lower. Thus if their spouse is already infected at the start of the marriage, an uninfected person may be at higher risk within marriage than before. On a population scale, early marriage can only be protective if the majority of newly weds of both sexes are virgins, or if they marry their first sexual partners.
In these six countries 27–81% of never married males under 25 years old are sexually active, as are 10–28% of unmarried females, with Zambia and Kenya showing the most extreme differences between the sexes. Overall, Uganda and Zimbabwe report low rates of premarital sexual activity; Kenya and Zambia have relatively high rates. The proportion of unmarried 15–24 year olds who are sexually active is problematic as a measure of “unsafe” sexual activity, as it does not allow for the effects of age structure in this age group. It is possible to construct life tables for entry into first marriage, and compare median ages at first sex and first marriage. However such a comparison would not distinguish between those who first experience sexual activity as a direct result of getting married, and those who have been sexually active before marriage.
To allow both for age structure effects and for the different paths that young people can follow to sexual activity and marriage, instead of simple two state life tables (virgins/sexually active; single/married) we can construct a multistate life table, allowing for virginity, sexual activity by those who are not married, and for entry into marriage from both of these states. Table 6 shows the person years spent between 15–24 years in these states by the two youngest 10 year birth cohorts for each country: those aged 25–34 years and those aged <25 years at the last survey. Ten year cohorts are used for this analysis because of the relatively low proportion married under age 20.
Person years lived between 15 and 25 by sexual activity status in the birth cohorts aged 25–34 years and 15–24 years at the last survey, by sex and country
Males spend much longer than females in the sexually active unmarried state in all countries. Larger proportions of women than men claim not to have been sexually active before marriage, so that females who have not had a previous sexual partner contribute many more person years of marriage in this risk group than do men. This implies that women would be exposed to higher risks of acquiring HIV from their husbands than vice versa even if after marriage neither partner had extramarital sex.
Uganda and Zambia have the highest proportions of women reporting they married as virgins; Kenya has the lowest. Uganda also has notably higher proportions of men who marry as virgins. Zambia and Kenya have very large differences between the sexes with respect to premarital sex; in Ghana the experience of males and females is fairly similar. High levels of premarital sexual activity are seen in Kenya and Zambia, with relatively low levels reported in Uganda and Zimbabwe. Uganda has much higher person years married (combining those who marry as virgins with those who are sexually experienced) than any other country, Kenya has the lowest (for both males and females).
The major intercohort changes for males are a large increase in virginity in Ghana (with a consequent lowering of premarital sexual activity) and an increase in premarital sexual activity in Zambia as a result of declines in transition from premarital sexual activity to marriage. Changes for females have been less dramatic—a general increase in virginity, most notably in Kenya and Uganda, and a decline in marriage among virgins in Ghana and Zambia, which has led to an increase in premarital sexual activity.
The striking between country and male/female differences in this multistate analysis are illustrated in figure 3, a series of stacked area graphs comparing the proportions of each year in the life table spent in each state. These diagrams show both the timing of the various transitions and the overall scale of “unsafe” premarital sexual activity in these populations. They also give an indication of the degree of risk faced by men and women within marriage, due to the past behaviour of the opposite sex. The countries compared are Uganda and Kenya, and the first graph in each series shows the experience of women aged 25–34 years at the time of the last survey, the middle panel illustrates the experience of women aged 15–24 years at that time, and the bottom panel refers to men aged 15–24 years.
Percentage of life table person years by sexual activity and marital status.
From the graphs it is clear that there is a bigger contrast between Uganda and Kenya in what happens after the start of sexual activity than in its timing. As seen in table 2, sexual initiation actually occurs earlier among Ugandan women than their counterparts in Kenya, but a higher proportion of this occurs as a result of early marriage. Among both men and women who experience sex before marriage in Uganda there is a fairly rapid transition to marriage, whereas in Kenya the period of premarital sexual activity is much longer, and involves a higher proportion of the cohort. There is a much more dramatic contrast between the experience of the sexes and between the two countries than between successive 10 year cohorts.
DISCUSSION
This analysis set out with a methodological and a substantive objective. Previous analyses of survey data on age at first sex have not made full use of available data. Survival analysis based on current status and recall data enhances sample size and provides more robust estimates of age at first sex. When multiple cross sectional surveys over time are available for the same population, analysis by birth cohort gives further insights into trends in age at first sex and into the effects of changes in the composition of the sample between surveys. The cohort approach revealed reporting biases that varied with age, which should be taken into account in trend analyses. Finally, it was demonstrated that a multistate life table is the most complete method to capture patterns of sexual initiation in relation to marriage.
The quality of self-reported data on initiation of sex was examined, with a focus on changes in bias with reporting age. Previous analyses of DHS data indicate that survey based aggregate measures of age at first sexual intercourse produce valid results,34 though some systematic errors in reporting of first sex have been suggested.35 In Zimbabwe men and women consistently report later initiation of sex than respondents in the other five countries, but this may be due in part to reporting bias caused by a denial of any kind of sexual activity by teenagers. The relatively limited exposure to premarital sex reported by adolescents in Zimbabwe is somewhat implausible in the context of the very high and rapidly rising level of HIV found in that country at the end of the 1990s, with both urban and rural sentinel clinics reporting prevalence of around 30% in pregnant women under 25.36
For evaluating interventions, changes in biases over time are the prime concern. If HIV prevention programmes strongly promote postponement of first sex, teenagers may become increasingly reluctant to report sexual activity. This may be particularly significant for those still in school, if strong sanctions exist against sexual activity for school children. Age may be misreported by some respondents who do not know their exact age, although this is likely to be less of a problem in adolescents and people in their early twenties than among the older population. There may however be deliberate misreporting of age under the influence of interventions. Teenagers who have admitted to sexual activity may tend to lie about their current age, making it difficult to trace true cohorts between surveys.
It is important to standardise for the educational composition of the cohorts being compared. Secondary education is a powerful determinant of age at first sex, and if there are changes in the proportions enrolled in secondary school it is useful to separate out the effects of such changes from overall secular changes affecting adolescents within educational subgroups.
The within-cohort comparisons, (fig 2 and table 4) and the statistical adjustment for education, residence, and reporting age (table 5) have alerted us to the wide range of biases that can affect a simple comparison of age at first sex from cross sectional surveys. A comparison of median age at first sex in successive age groups, or in succeeding surveys can make it appear that adolescent sexual behaviour is changing when the differences are actually due to reporting errors or sampling problems.
This paper has suggested a variety of ways for identifying and allowing for these biases, and has shown that in both Kenya and Uganda, the positive findings about a later start to sexual activity stand up to critical scrutiny. The changes in Uganda appear to be larger than in other countries, but women in Uganda had more scope for change because historically they had earlier initiation of sexual activity. Our proposed extension of the life table approach to quantifying the contribution of premarital sexual activity to sexual risk exposure in adolescence also highlighted the potential importance of the length of the transition period between start of sexual activity and marriage among both sexes as a factor to be considered when explaining HIV trends.
Substantively, the key issue was to examine whether Uganda’s trends in adolescent sexual activity were different from those of other countries. The focus on Uganda was prompted by the unique course of the HIV epidemic in that country: the early start, with evidence of substantial numbers of infections in the late 1970s, and steady escalation throughout the 1980s—a time of civil unrest, war, and associated movements of troops and refugees. Since then Uganda has been the only country in sub-Saharan Africa with a significant, sustained, nationwide decline in HIV prevalence during the 1990s.37 In contrast, the epidemic is still rising, or at best stationary in other badly affected countries of eastern and southern Africa.38 Relatively little has been written about the causes of the early rise in HIV in Uganda, but there has been a great deal of speculation about the role of delayed sexual debut among teenagers as an explanatory factor of the decline.39
Table 5 showed that statistically significant increases in age at first sex had indeed occurred in Uganda for both males and females, throughout the 1990s, although the trends found in the DHS analysis were smaller than those reported in a study of two urban areas in Uganda.4 However, Ghana and Kenya also recorded significant increases in age at first sex among women, with little concomitant change in HIV prevalence. The changes in Uganda happened against a historical background of very early sexual initiation, largely a result of early marriage, and Uganda has maintained a rapid transition from sexual activity to marriage for both sexes, whereas Kenya continues to have a relatively slow transition. Zambia has experienced the worst type of change among males: a lengthening of the time spent sexually active but unmarried. This is a pattern which is dangerous both for the young men, and for the women whom they eventually marry.
CONTRIBUTORS
EP began the process by uncovering discrepancies during her studies of DHS data, JTB conceived the idea for a comparative analysis, BZ devised the analytical procedures, wrote the computer programs, and interpreted the results, ES adapted and re-ran the procedures to incorporate the latest data from Zambia, and all four collaborated in writing the text.