Article Text
Abstract
Primary and secondary prevention are essential components of the response to HIV and sexually transmitted infections (STIs). We present findings from nationally implemented HIV/STI prevention interventions. In 2003, of those attending STI clinics at least 64% of men who have sex with men (MSM) and 55% of heterosexuals accepted a confidential HIV test; 88% of all HIV infections in women giving birth in England were diagnosed before delivery; 85% of MSM eligible for hepatitis B vaccination received a first dose of vaccine at their first STI clinic attendance; 74% of STI clinic attendees for emergency appointments, and 20% of those for routine appointments were seen within 48 hours of initiating an appointment; the National Chlamydia Screening Programme in England found a positivity of 10% and 13% among young asymptomatic women and men, respectively. Prevention initiatives have seen recent successes in limiting further HIV/STI transmission. However, more work is required if current levels of transmission are to be reduced.
- ARV, antiretroviral therapy
- IDUs, injecting drug users
- MSM, men who have sex with men
- NCSP, National Chlamydia Screening Programme
- NSHPC, National Study of HIV in Pregnancy and Childhood
- SOPHID, Survey of Prevalent HIV Infections Diagnosed
- STI, sexually transmitted infections
- UAPMP, Unlinked Anonymous Prevalence Monitoring Programme
- VCT, voluntary confidential HIV testing
- sexually transmitted infections
- HIV
- United Kingdom
Statistics from Altmetric.com
- ARV, antiretroviral therapy
- IDUs, injecting drug users
- MSM, men who have sex with men
- NCSP, National Chlamydia Screening Programme
- NSHPC, National Study of HIV in Pregnancy and Childhood
- SOPHID, Survey of Prevalent HIV Infections Diagnosed
- STI, sexually transmitted infections
- UAPMP, Unlinked Anonymous Prevalence Monitoring Programme
- VCT, voluntary confidential HIV testing
Prevention is an essential component of the response to HIV and sexually transmitted infection (STI) transmission. Despite the availability of effective antiretroviral therapy (ARV), HIV infection is only treatable provided that an assiduous routine of medication is followed indefinitely (often with adverse side effects1). HIV care and treatment are expensive2 and ARV resistance is thought to be increasing in England.3 STI treatment costs are also substantial4 and if left untreated, can have serious long term sequelae5 and possibly facilitate the transmission of others, including HIV.6
For HIV/STIs, primary prevention targets uninfected individuals, for instance, by reducing risk factors for disease acquisition. Examples include diagnosing HIV in pregnant women (to prevent vertical transmission), hepatitis B vaccination, and harm reduction measures (for example, needle exchanges). Secondary prevention targets infected individuals, aiming to reduce onward disease transmission or re-infection. Examples include the promotion of sexual health screening—for example, chlamydia screening among young people and promoting voluntary confidential HIV testing (VCT) in STI clinics. An HIV diagnosis provides access to ARV, a timely STI diagnosis usually leads to treatment, and both allow an opportunity for partner notification and behaviour change counselling.
Major challenges remain in ensuring that prevention initiatives are effective. They need to be accessible (particularly for higher risk populations who may be socially vulnerable); timely; comprehensive (address all modes of HIV/STI transmission); implemented through functioning health systems; and subject to monitoring and evaluation.
The Health Protection Agency and its collaborators monitor the effectiveness of some primary and secondary prevention efforts in addition to providing national HIV/STI surveillance data. We present findings from nationally coordinated prevention monitoring programmes. This paper does not present an overview of all HIV/STI prevention activities that occur in the United Kingdom, but summarises information on prevention monitoring and disease outcomes, to demonstrate recent progress, and highlight areas that need further work.
DATA SOURCES
In the United Kingdom, the majority of HIV/STI prevention initiatives are implemented through STI clinics, primary care, and other community based services. The Health Protection Agency and its collaborators use nationally coordinated information systems to monitor prevention initiatives. Prevention monitoring systems7 (summarised in table 1) and their objectives2,8 are generally separate from the variety of infection surveillance systems used, but can overlap.
Summary of aims and outcomes of prevention interventions nationally monitored by the Health Protection Agency and its collaborators*
HIV testing
Monitoring the uptake of VCT and antenatal HIV screening relies on data from the Unlinked Anonymous Prevalence Monitoring Programme (UAPMP) surveys.9
The UAPMP survey of STI clinic attendees measures HIV prevalence (including undiagnosed HIV infection) in attendees of 16/232 STI clinics in England, Wales, and Northern Ireland undergoing syphilis tests.10 Residual blood from syphilis testing is irreversibly unlinked from patient identifiers and anonymously HIV tested. Retained information includes sexual orientation and sexual health screen uptake (including VCT, further details are available on the STI website).
The UAPMP surveys of pregnant women (utilising residual serum from newborn infant dried blood spots, covering 80% of births in England and Scotland) provide a proxy measure of HIV prevalence in the overall population. Live births to diagnosed HIV infected women in the United Kingdom are reported to the National Study of HIV in Pregnancy and Childhood (NSHPC).8 The proportion of HIV infected women diagnosed before delivery is calculated by aligning NSHPC reports11,12 with the total number of births to diagnosed and undiagnosed HIV infected women. The number of infants who become infected themselves is estimated by applying UK specific observed transmission rates for infants born to diagnosed and undiagnosed HIV infected women.13
CD4 surveillance
CD4 T lymphocyte counts in HIV infected individuals (CD4 Surveillance Scheme) are reported from 60 laboratories in England and Wales (representing approximately two thirds of all reported new diagnoses) and are used to monitor trends in immunosuppression at HIV diagnosis.14 Individuals who have a CD4 count below 200 cells ×106/l (the recommended threshold for beginning therapy15) at HIV diagnosis are categorised as having a “late HIV diagnosis.”
Antiretroviral therapy monitoring among diagnosed HIV infected individuals
The annual Survey of Prevalent HIV Infections Diagnosed (SOPHID) provides a census of the total number of individuals receiving HIV related care in England, Wales and Northern Ireland.16,17 Subsidiary information is collected on ARV uptake and most recent CD4 count.
Uptake of hepatitis B vaccination among men who have sex with men
The HepB3 survey monitors hepatitis B vaccination uptake among eligible men who have sex with men (MSM) on their first STI clinic attendance. Since the study started in 2003, 187/209 English clinics have participated.
Chlamydia screening programme
The National Chlamydia Screening Programme (NCSP) aims to control genital chlamydial infection through early detection of asymptomatic infection18,19 outside STI clinic settings. From April 2003 to March 2004, 302 screening venues (including contraceptive clinics, GPs, young people’s services, and termination clinics) participated from 10 programme areas. The target population is sexually active individuals aged under 25. Demographic and behavioural data are also collected.
STI clinic waiting times
Since 2004, a biannual audit of waiting times is conducted among all new attendees at all STI clinics in England for 1 week. Age and sex specific waiting times are collected for each clinic as discreet categorical units.
Behavioural and serosurveillance of injecting drug users
The UAPMP survey of injecting drug users (IDUs) collects self reported behavioural data (for example, injecting equipment sharing) in addition to measuring the prevalence of blood borne viruses among injectors attending 63 specialist services in England, Wales, and Northern Ireland.20 Sharing rates are calculated for those who reported injecting in the previous 4 weeks.
Descriptive epidemiology is the focus of the paper, but 95% confidence limits (95% CI) have been used to supplement main findings from the NCSP and the sentinel Unlinked Anonymous STI and IDU survey. All other prevention monitoring systems are comprehensive.
RESULTS: PREVENTION MONITORING UPDATE
VCT uptake
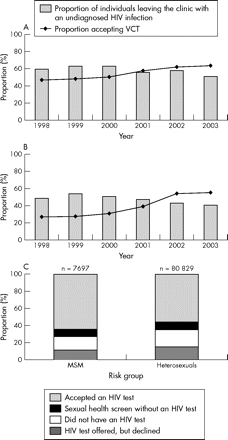
Overall, VCT uptake rose by 17% (95% CI 15% to 19%) from 47% (2956/6294) in 1998 to at least 64% (4920/7697) in 2003 among MSM and by 28% (95% CI 27% to 28%) from 27% (16 886/62 295) in 1998 to at least 55% (44 312/80 435) among heterosexuals (fig 1A and B). Of those who did not have VCT, at least 29% (817/2777) of MSM and 31% (11 312/36 123) of heterosexuals are known to have been offered, but declined, VCT (fig 1C). Of those that declined VCT, 7% (56/817) of MSM and 1% (83/11 312) of heterosexuals were HIV infected.
Proportion* of STI clinic attendees, accepting VCT†, and proportion of HIV infected individuals who leave the clinic with their HIV infection remaining undiagnosed, England, Wales, and Northern Ireland 1998–2003. (A) MSM, (B) heterosexuals, (C) proportion* of STI clinic attendees accepting VCT† by exposure category, 2003. (*Excludes HIV infected individuals who were previously diagnosed. Patients for whom conflicting information was provided on HIV testing were excluded. †Attending 16 GUM clinics in England, Wales, and Northern Ireland.) Data source: Unlinked anonymous programme.
The proportion of HIV infected individuals who could have been diagnosed during their attendance, but who left the clinic remaining unaware of their HIV infection, fell by 9% (95% CI 1% to 17%) from 60% (165/276) in 1998 to 51% (161/317) in 2003 among MSM and may have fallen by 7% (95% CI −1% to 15%) from 48% (104/217) in 1998 to 41% (160/394) in 2003 among heterosexuals.
Late diagnoses
In 2003, 33% (995/2982) of people with newly diagnosed HIV infection in England and Wales had CD4 counts below 200 cells ×106/l. MSM are increasingly being tested at earlier stages of their infection21 (fig 2). HIV infected heterosexuals were more likely to be diagnosed late.
Late diagnosis* of HIV infection by exposure category, England and Wales, 1994–2003. (*Percentage of patients with CD4 count under 200 cells ×106/l within 90 days of diagnosis.) Data source: CD4 surveillance scheme.
Antenatal HIV testing
In England, 88% (697/790) of HIV infected women who gave birth in 2003 are calculated to have had their infection diagnosed before delivery. The majority of births to HIV infected women occurred in London where 89% (455/511) of women were diagnosed before delivery (fig 3).
Estimated proportion of HIV infected women diagnosed before delivery*, and of exposed children becoming HIV infected†, England, 1998–2003. (*Includes previously diagnosed and those diagnosed through antenatal testing. †Assumes a vertical transmission rate of 26.5% in undiagnosed women and 2.2% in diagnosed women.13 ‡These data contain reports received by the end of September 2004 and are subject to reporting delay, particularly for 2003.) Data source: Unlinked Anonymous Programme and the National Study of HIV in Pregnancy and Childhood.
The proportion of children exposed to maternal HIV infection who acquire HIV is decreasing. Based on current estimated detection rates, 5% of children exposed to vertical transmission would have been infected in England in 2003, compared to 17% in 1998.
Uptake of antiretroviral therapy
Of MSM receiving HIV care, 65% (9991) were receiving at least three antiretroviral drugs in 2003 compared to 56% (5231) in 1998. Among heterosexuals, equivalent figures were 63% (9956) and 50% (2158). Thirty three per cent of MSM (5065) and 35% (5466) of heterosexuals were not receiving HIV therapy in 2003.
Uptake of hepatitis B vaccine among MSM
MSM were considered to be eligible for hepatitis B vaccination (dose 1) if they were not known to be either immune or fully/partially vaccinated. Overall, 85% (5598/6553) of eligible MSM were vaccinated with dose 1.
MSM eligible for the third vaccine dose (dose 3) included those who had had fewer than three doses, but excluded those who had had a booster dose, and those known to have immunity through blood testing following previous doses. The coverage rate for dose three was 39% (2588/6624) overall but showed regional variation (fig 4). Nearly one half (46%, 2588/5669) of MSM eligible for dose 1 completed the three dose course.
Coverage rates for hepatitis B vaccination (first and third dose) among eligible MSM attending STI clinics for the first time, by region, England, 2003. Data source: HepB3 survey.
STI clinic waiting times
Nationally, 74% (1359/1843) of emergency appointments, 79% (4960/6307) of people attending walk-in clinics, and 20% (3044/15 520) of people with routine appointments were seen within 48 hours. Lower proportions of 16–24 year olds and women of any age were seen within 48 hours. Full results have been published elsewhere.22
Chlamydia screening programme
In England, 16 413 young people were screened for chlamydia outside STI clinic settings during April 2003–March 2004.19 A 10% (1538/15 241, 95% CI 9.6% to 10.6%) and 13% (156/1172, 95% CI 11.4% to 15.4%) positivity among women and men aged under 25 was found respectively (fig 5). Women aged 16–19 were more likely to test positive for chlamydia than those aged 20–24; men aged 20–24 were twice as likely to be infected as younger men.
Chlamydia positivity, opportunistic screening outside STI clinics by sex and age group, England, April 2003–March 2004. Data source: National Chlamydia Screening Programme.
Risk behaviours among IDUs
Thirty per cent (494/1677, 95% CI 28% to 32%) of injectors reported sharing needles and syringes in 2003; a level similar to that observed since 1998.23 In 2003, 85% (331/384) of IDUs who had first injected in the previous 3 years reported ever having accessed a needle exchange service.
DISCUSSION
Monitoring individual HIV/STI prevention initiatives in 2003 demonstrated successes. VCT uptake among STI clinic attendees was at the highest level ever recorded. Almost 90% of HIV infected women had their infection diagnosed before delivery in England leading to an increase in the proportion able to take advantage of interventions to prevent vertical transmission (for example, ARV and avoiding breast feeding), thereby decreasing the proportion of infants who become infected. The proportion of diagnosed HIV infected individuals on at least triple therapy has increased from around half in 1998 to almost two thirds in 2003. HIV infected MSM are being diagnosed at an earlier stage of their infection. Targeted screening for chlamydia has identified many asymptomatic infections that otherwise may have been missed.
However, high proportions of STI clinic attendees were not seen within 48 hours of seeking an appointment, delaying access to treatment and potentially heightening the risk of onward transmission.24 The elevated levels of injecting equipment sharing among IDUs is concerning.
Are prevention initiatives reducing transmission?
Despite the individual success of many prevention initiatives, the number of HIV/STI diagnoses are increasing annually. In 2003 there were 6606 new HIV diagnoses, more than double the 2835 diagnoses in 1998.7 From 2002 to 2003, diagnoses of chlamydia at STI clinics rose by 8%, and syphilis by 28% among men and 32% among women.7
The number of diagnoses is determined by interactions between transmission dynamics, migration of individuals from high prevalence areas, and the relative effectiveness of targeted testing. Increases in diagnoses represent both successes in increased testing in high risk populations as well as continued disease transmission within that population. The relative contribution of these factors is difficult to disentangle.
However, HIV incidence may be increasing among MSM attending STI clinics,25 whilst HIV prevalence among recently initiated injectors (those who began injecting in the previous 3 years) was at the highest level recorded in 2003, perhaps a result of elevated levels of equipment sharing.20
The reasons for continuing transmission despite apparently successful prevention initiatives require consideration:
Do prevention monitoring systems show the whole picture?
In the United Kingdom, methods for monitoring prevention initiatives are pragmatic, simple, inexpensive and reach accessible populations—not necessarily those at greatest risk. Consequently, data are generally derived from those using health services, and will exclude high risk populations who have poor access to services.
Monitoring systems also collect limited data. While rates of STIs are higher among black and ethnic minority populations,26,27 few UK prevention monitoring systems collect ethnicity data, making it difficult to monitor the specific impact of prevention initiatives on such vulnerable populations.
Each prevention monitoring system has its own limitations.
HIV testing
All patients included in the Unlinked Anonymous STI survey are undergoing syphilis tests, therefore, VCT in this population may not represent all STI clinic attendees. The survey cannot monitor the frequency of repeat HIV testing (which may positively bias the results), or outcomes among first time attendees.
Diagnosis detection rates among HIV infected pregnant women are calculated by aligning diagnosis reports to the NSHPC with UAPMP prevalence data. Since data are anonymised, records are not individually matched. Limited mismatching may occur with respect to time and geography. Detection rates are minimum estimates and may rise as late reports are received by the NSHPC.
CD4 surveillance suggests MSM with HIV are being diagnosed earlier in their infection. However, the high proportion of heterosexuals categorised as having a “late diagnoses” may not accurately reflect recent efforts in VCT promotion; a high proportion of heterosexuals are infected abroad and may not have been resident in the United Kingdom long enough to have had an earlier diagnosis.
ARV
HIV infected individuals accessing health care show an increase in ARV uptake. However, it is difficult to calculate what proportion of HIV infected individuals should be on therapy. Guidelines state that individuals who have a CD4 count below 200 cells ×106/l should begin therapy.15 Such guidelines are not directed from a public health perspective to prevent HIV transmission, but on the basis of individual need/readiness; patients may delay, interrupt, or stop therapy for many reasons.
The proportion of diagnosed HIV infected individuals with low CD4 counts on ARV is not routinely calculated because these specific fields are incomplete for a minority of records. In the future, this proportion may be calculated through cross linking to other HIV reporting databases.28
Hepatitis B vaccination
The HepB3 study demonstrates that high proportions of MSM are vaccinated with dose 1 on their first STI clinic attendance, but lower proportions complete the three dose course. Patient identifiers are not collected, so it is impossible to monitor movement of patients between clinics. This, combined with reporting delay, may lead to an underestimate of the true performance.
STI clinic waiting times
Although the national waiting times survey show a high proportion of attendees cannot get a timely appointment, it is not possible to calculate the median waiting time since data is collected in categories.
Chlamydia screening programme
While the NCSP has improved access for chlamydia screening since its implementation in England, it is not currently possible to calculate national coverage. When the programme is fully rolled out throughout England, coverage will be calculated by dividing the number of people aged 16–24 screened by the total eligible population (sexually active population aged 16–24).
Risk behaviour monitoring
The Unlinked Anonymous IDU survey only includes those in contact with services for drug users and therefore the data may not be generalisable to all injectors, specifically, those not in contact with services, who may have different levels of risk behaviour.
Are prevention initiatives effective?
The effectiveness of prevention initiatives to reduce transmission requires assessment. For instance, although the promotion of VCT has reduced the proportion of HIV infected individuals leaving the clinic remaining undiagnosed, it may not target those who have been recently infected, who may be more infectious. Research is required to elucidate the role of “recently HIV infected” people in contributing onward HIV transmission. For IDUs, there is evidence of a recent shift in needle exchange provision towards pharmacy based services.29 Studies suggest that IDUs using pharmacy based services may be more likely to share equipment owing to absence of harm reduction counselling in these settings.30
Are prevention initiatives implemented on the correct scale?
Since the prevalence of an infection drives onward transmission, recent increases in the prevalent pool of diagnosed and undiagnosed infections may limit the ability of prevention programmes to reduce transmission levels.
For example, the success in reducing the proportion of infants exposed to maternal HIV infection who become infected, has not substantially reduced the absolute number, because prevalence among pregnant women has increased. Continuing investment in prevention activities is essential, and activities need to adapt to meet the challenge of the evolving epidemics.
What next?
While individual prevention initiatives have had an impact, and rates of ongoing transmission would have been higher in their absence, investment needs to be strengthened and sustained in order to reduce HIV/STI transmission. Current prevention initiatives partially accommodate the diversity of populations at high risk of infection, but require flexibility if they are to match the evolving epidemic. Evaluation of prevention initiatives, both individually and in combination, is needed to measure how much they reduce transmission. Novel monitoring tools require development to assess prevention initiatives aimed at populations who have poor access to health services, but who play an important part in HIV/STI transmission.
Local and national surveillance systems are essential in ensuring that the effectiveness of prevention initiatives are continually reviewed and updated to meet the diversity of needs of the populations at risk of HIV/STIs.
Key messages
-
Prevention activities have seen recent successes in limiting further HIV/STI transmission in England, Wales, and Northern Ireland
-
High proportions of STI clinic attendees are having voluntary confidential HIV tests. HIV infected pregnant women are diagnosed before delivery; MSM are receiving hepatitis B vaccinations at their first clinic attendance
-
Prevention monitoring activities may be missing at-risk populations who are likely to have an important role in onward HIV/STI transmission
-
Existing prevention initiatives may only be having a limited effect on the current rate of HIV/STI transmission and require development to match evolving epidemics
Acknowledgments
We thank David Goldberg, Daniel Thomas, Brian Smyth, Sarah Dougan, Elizabeth Rudd, Christine McGarrigle, and all others who contributed to the writing and editing of the annual report Focus on Prevention.
We gratefully acknowledge the continuing collaboration of the Sexually Transmitted and Blood-Borne Viruses Laboratory, Centre for Infections, Health Protection Agency and of clinicians, microbiologists, immunologists, public health practitioners, midwives, and other colleagues who contribute to the surveillance of HIV/STIs in the United Kingdom. We are also grateful to the English Department of Health for funding specific surveys.
We would like to thank our collaborating centres for HIV and AIDS surveillance in the UK: Health Protection, Scotland; The Institute of Child Health (London); The UK Haemophilia Centres Doctors Organisation; members of the Scottish ISD(D)5 Collaborative Group; Collaborators on the Unlinked Anonymous Programme (a full list of collaborators available at: www.hpa.org.uk/infections/topics_az/hiv_and_sti/hiv/epidemiology/ua.htm).
Confidential reports of HIV infected pregnant women are collated at the Institute of Child Health by the National Study of HIV in Pregnancy and Childhood through surveillance schemes run in collaboration with the Royal College of Obstetricians and Gynaecologists and the British Paediatric Surveillance Unit of the Royal College of Paediatrics and Child Health. Research at the Institute of Child Health benefits from R&D funding received from the NHS Executive.
Finally, we thank Dr Helen Ward and Susie Huntington for their useful comments on drafts of this paper.
CONTRIBUTORS AB, LL, SL, HM, VH, AR, BR, and TC analysed the data from the Unlinked Anonymous STI clinic survey, Unlinked Anonymous pregnant women survey, National Chlamydia Screening Programme, HepB3 survey, Unlinked Anonymous IDU survey, STI waiting times survey, SOPHID and CD4 surveillance scheme respectively with support from VD, NG, and KF; PT coordinates the National Study of HIV in Pregnancy and Childhood and collaborated with the analysis of the Unlinked Anonymous Pregnant Women Surveys; VD oversees the data for SOPHID, CD4 surveillance, and the NCSP; NG is the programme manager and is responsible for data from the Unlinked Anonymous Programme; JP is responsible for laboratory aspects for the Unlinked Anonymous Surveys and assisted with the interpretation of data; all authors were involved in interpretation of the results and drafting the paper with ST and JB substantially contributing; AB undertook the main writing of the paper.
REFERENCES
Supplementary materials
Files in this Data Supplement: