-
PDF
- Split View
-
Views
-
Cite
Cite
David I. Bernstein, Abbie R. Bellamy, Edward W. Hook, Myron J. Levin, Anna Wald, Marian G. Ewell, Peter A. Wolff, Carolyn D. Deal, Thomas C. Heineman, Gary Dubin, Robert B. Belshe, Epidemiology, Clinical Presentation, and Antibody Response to Primary Infection With Herpes Simplex Virus Type 1 and Type 2 in Young Women, Clinical Infectious Diseases, Volume 56, Issue 3, 1 February 2013, Pages 344–351, https://doi.org/10.1093/cid/cis891
Close - Share Icon Share
Abstract
Background. Herpes simplex virus infections type 1 (HSV-1) and type 2 (HSV-2) are common, but the epidemiology of HSV disease is changing.
Methods. HSV-seronegative women, aged 18–30 years, who were in the control arm of the HERPEVAC Trial for Women were followed for 20 months for primary HSV infections.
Results. Of the 3438 evaluable participants, 183 became infected with HSV: 127 (3.7%) with HSV-1 and 56 (1.6%) with HSV-2. The rate of infection for HSV-1 (2.5 per 100 person-years) was more than twice that for HSV-2 (1.1 per 100 person-years). Most infections (74% of HSV-1 and 63% of HSV-2) occurred without recognized signs or symptoms of herpes disease. The HSV-2 infection rate was 2.6 times higher in non-Hispanic black participants than in Hispanics and 5.5 times higher than in non-Hispanic whites (P < .001), while the HSV-1 infection rate was 1.7 times higher in non-Hispanic whites than non-Hispanic blacks. Younger participants (18–22 years) were more likely to acquire HSV-1 infections and less likely to develop recognized disease than older participants. Overall, 84% of recognized disease cases were genital. No differences were noted in the clinical manifestations of genital HSV-1 vs genital HSV-2 disease. The clinicians’ assessment that cases were caused by HSV was good when they assessed cases as clinically confirmed or unlikely (validated in 83% and 100% of cases, respectively).
Conclusions. HSV-1 is now more common than HSV-2 as a cause of oral and genital mucosal infections in young women, but there are important age and race differences.
(See the Editorial Commentary by Whitley on pages 352–3.)
Herpes simplex virus (HSV) is a common cause of both genital and oral disease. HSV type 2 (HSV-2), a sexually transmitted pathogen, infects >500 million people worldwide and causes an estimated 23 million new infections each year [1]. HSV type 1 (HSV-1) is even more common, with an estimated seroprevalence of >90% in many nations [2]. HSV-1 is frequently acquired during early childhood, primarily through oral secretions [3]. However, the epidemiology of HSV-1 is changing, such that the frequency of sexual transmission of HSV-1 has increased in many countries, including the United States [4, 5].
The control arm of an investigational HSV-2 vaccine study, the HERPEVAC Trial for Women [6], provided the opportunity to prospectively follow a large cohort of HSV-seronegative women in order to characterize the epidemiology, clinical manifestations, and antibody response to primary HSV infections. Thus, all infections identified in these women were primary infections in which the etiology (HSV-1 or HSV-2) could be confirmed by Western blot analysis and the identification of symptomatic vs unrecognized primary infections categorized with a high degree of certainty. This obviated limitations present in past studies where primary and recurrent infections might be misclassified, and where the ability to serologically distinguish HSV-1 from HSV-2, was suboptimal.
METHODS
Study Population
A subset of participants enrolled in the HERPEVAC Trial for Women, a phase 3 herpes vaccine study [6] at 50 sites in the United States and Canada from 2003 to 2007, were included in this analysis. Women aged 18–30 years who were seronegative for HSV-1 and HSV-2, absent of significant health problems, not pregnant, and willing to use effective methods of birth control, were enrolled. The analysis presented here includes the 3438 women who only received the control hepatitis A vaccine (Havrix) in this trial, and had at least 1 follow-up visit. Race and ethnicity were self-described by participants and classified as non-Hispanic white, non-Hispanic black, or Hispanic (any race) for analysis. Women with missing data or not fitting into one of these categories were classified as “other.”
Study Design
Participants were followed for 20 months after study entry. They were educated regarding the signs and symptoms of genital and nongenital herpes disease and requested to attend clinic within 48 hours, or as soon as possible thereafter, if they noted possible signs or symptoms. Active surveillance for suspected HSV disease was conducted monthly by telephone call, e-mail, text message, or social media communication. Serum for evaluation of infection (seroconversion) was obtained prior to vaccination (month 0) and at months 2, 6, 7, 12, 16, and 20. At clinic visits for suspected genital or nongenital herpes disease, participants were examined, viral cultures were obtained, and treatment was initiated at the discretion of the local investigator. The protocol was approved by the institutional review boards/ethics committees at all sites, and participants gave written informed consent.
An HSV infection was defined as unrecognized if a woman seroconverted to HSV-1 or HSV-2 and did not present with any signs or symptoms of disease within the previous 6 months. HSV disease was defined by the presence of compatible signs and/or symptoms with confirmation by virus culture and/or seroconversion within 6 months after onset of symptoms. Cases were determined centrally by an independent blinded endpoint review committee.
Laboratory Methods
Serum samples were evaluated for antibodies to HSV-2 with HerpeSelect-2 (Focus Technologies, Cypress, CA) after each blood draw. Samples testing positive, using the manufacturer's criteria, were then tested for HSV-1 and HSV-2 antibody using Western blot (WB) at the University of Washington [7]. For each participant, the first and last serum samples were also tested by WB. Seroconversion was defined as a positive HSV-1 and/or HSV-2 WB in a participant with a previously negative result for the corresponding HSV type. If seroconversion was detected using the first and last sample, all samples were tested to determine the time of seroconversion.
Statistical Analysis
Categorical variables were compared by Fisher exact test, and continuous variables were compared by Student t test. Cox proportional hazards models were used to compare rates of HSV-1 and HSV-2 infection by age group and race/ethnicity.
The association between genital HSV disease and participants’ reported signs and symptoms was assessed by fitting separate univariate logistic regression models for each sign or symptom (lesion location [genital or perianal/buttocks], papules, vesicles, ulcers, crusts, fissures, pain, painful urination, redness, vaginal discharge, swelling, headache, malaise, muscle ache, fever). Then, any sign or symptom found to be significant (P < .05) in the univariate models was considered for inclusion in a multivariate logistic regression model. Signs and symptoms were chosen for inclusion in the multivariate model using a forward selection algorithm; where at each step the variable that most reduced Akaike information criterion was selected. The variables selected by the model were used to define classification rules for genital HSV disease, and the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of each rule compared with the participant's true HSV genital disease status was calculated. The logistic regression modeling analyses included 165 participants who presented with at least 1 suspected genital herpes episode and provided complete diary card data; 17 participants without completed diary cards were excluded.
RESULTS
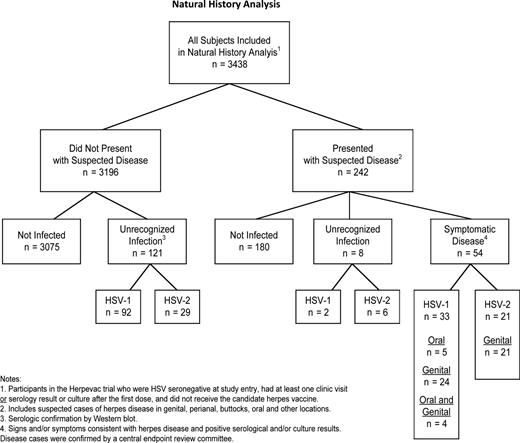
Of the 3438 subjects included in the natural history analysis, 98 (3%) did not have any samples tested by WB, 2 (<1%) had only baseline WB results, and 3338 (97%) had first and last samples tested by WB. Of these 3438 participants, 183 became infected with HSV: 127 (3.7%) with HSV-1 and 56 (1.6%) with HSV-2. Of the 242 women who presented for evaluation of signs and/or symptoms that the participant felt were compatible with HSV infection, as they had been instructed, 54 (22%) had confirmed HSV disease (Figure 1). Irrespective of virus type, most (84%) recognized disease was genital. Of the 54 participants with symptomatic HSV, 33 had HSV-1 disease (5 oral, 24 genital, 4 both genital and oral), and 21 had HSV-2 disease (all genital). Two additional participants from this group who were not infected at the time of their clinical evaluation later developed unrecognized HSV-1 infections, while 6 developed unrecognized HSV-2 infections (Figure 1). An additional 92 participants who did not present with suspected disease became infected with HSV-1, while 29 who did not present with suspected disease became infected with HSV-2. Thus, most infections in this study (74% of HSV-1 and 63% of HSV-2) occurred without recognized signs or symptoms of herpes disease despite frequent reminders to return to the clinics with any sign or symptom compatible with HSV disease. The rate of infection for HSV-1 was 2.5 cases per 100 person-years (py) and for HSV-2, 1.1 cases per 100 py (Table 1). Among non-Hispanic black participants, 74% (20 of 27) of those who acquired HSV were found to have HSV-2 (rate of infection, 4.4 per 100 py). In comparison, HSV-2 accounted for 23% (31 of 135) of HSV infections in non-Hispanic white participants (rate of infection, 0.8 per 100 py; P < .0001 vs non-Hispanic black participants). In Hispanic participants with HSV infections, 40% (4 of 10) were HSV-2 (attack rate, 1.7 per 100 py), while 9% (1 of 11) of HSV infections in participants of other race/ethnicity were HSV-2. In contrast, the rate of infection for HSV-1 was 1.5 per 100 py in non-Hispanic blacks, and 2.6 per 100 py in both non-Hispanic whites and in Hispanics. Participants 18–22 years of age were significantly more likely than older participants to acquire HSV-1 infections and significantly more likely to develop asymptomatic or unrecognized disease. This trend was similar for HSV-2 infections, although the differences were not significant.
Rate and Frequency of Herpes Simplex Virus Infection in Healthy Young Women
| . | . | Rate per 100 py (No. of Cases) . | |||
|---|---|---|---|---|---|
| Infection Type . | No. . | All Infections . | Unrecognized Infection . | Genital Disease . | Oral Disease . |
| HSV 1 | |||||
| All subjects | 3438 | 2.5 (127) | 1.9 (94) | 0.5 (28) | 0.2 (9) |
| 18–22 y | 1928 | 3.2 (89) | 2.5 (71) | 0.5 (15) | 0.2 (5) |
| 23–26 y | 1063 | 1.6 (26)c | 1.0 (16)a | 0.5 (8) | 0.2 (3) |
| 27–30 y | 447 | 1.8 (12) | 1.0 (7)b | 0.7 (5) | 0.1 (1) |
| Non-Hispanic white | 2693 | 2.6 (104) | 1.9 (76) | 0.6 (24) | 0.2 (8) |
| Non-Hispanic black | 342 | 1.5 (7) | 1.1 (5) | 0.4 (2) | 0.0 (0) |
| Hispanic | 168 | 2.6 (6) | 1.7 (4) | 0.4 (1) | 0.4 (1) |
| Other | 235 | 3.0 (10) | 2.7 (9) | 0.3 (1) | 0.0 (0) |
| HSV 2 | |||||
| All subjects | 3438 | 1.1 (56) | 0.7 (35) | 0.4 (21) | |
| 18–22 y | 1928 | 1.3 (37) | 0.8 (22) | 0.5 (15) | |
| 23–26 y | 1063 | 0.8 (13) | 0.5 (8) | 0.3 (5) | |
| 27–30 y | 447 | 0.9 (6) | 0.7 (5) | 0.1 (1) | |
| Non-Hispanic white | 2693 | 0.8 (31) | 0.5 (21) | 0.2 (10) | |
| Non-Hispanic black | 342 | 4.4 (20)d | 2.6 (12)d | 1.7 (8)d | |
| Hispanic | 168 | 1.7 (4) | 0.8 (2) | 0.8 (2) | |
| Other | 235 | 0.3 (1) | 0.0 (0) | 0.3 (1) | |
| . | . | Rate per 100 py (No. of Cases) . | |||
|---|---|---|---|---|---|
| Infection Type . | No. . | All Infections . | Unrecognized Infection . | Genital Disease . | Oral Disease . |
| HSV 1 | |||||
| All subjects | 3438 | 2.5 (127) | 1.9 (94) | 0.5 (28) | 0.2 (9) |
| 18–22 y | 1928 | 3.2 (89) | 2.5 (71) | 0.5 (15) | 0.2 (5) |
| 23–26 y | 1063 | 1.6 (26)c | 1.0 (16)a | 0.5 (8) | 0.2 (3) |
| 27–30 y | 447 | 1.8 (12) | 1.0 (7)b | 0.7 (5) | 0.1 (1) |
| Non-Hispanic white | 2693 | 2.6 (104) | 1.9 (76) | 0.6 (24) | 0.2 (8) |
| Non-Hispanic black | 342 | 1.5 (7) | 1.1 (5) | 0.4 (2) | 0.0 (0) |
| Hispanic | 168 | 2.6 (6) | 1.7 (4) | 0.4 (1) | 0.4 (1) |
| Other | 235 | 3.0 (10) | 2.7 (9) | 0.3 (1) | 0.0 (0) |
| HSV 2 | |||||
| All subjects | 3438 | 1.1 (56) | 0.7 (35) | 0.4 (21) | |
| 18–22 y | 1928 | 1.3 (37) | 0.8 (22) | 0.5 (15) | |
| 23–26 y | 1063 | 0.8 (13) | 0.5 (8) | 0.3 (5) | |
| 27–30 y | 447 | 0.9 (6) | 0.7 (5) | 0.1 (1) | |
| Non-Hispanic white | 2693 | 0.8 (31) | 0.5 (21) | 0.2 (10) | |
| Non-Hispanic black | 342 | 4.4 (20)d | 2.6 (12)d | 1.7 (8)d | |
| Hispanic | 168 | 1.7 (4) | 0.8 (2) | 0.8 (2) | |
| Other | 235 | 0.3 (1) | 0.0 (0) | 0.3 (1) | |
Abbreviations: HSV, herpes simplex virus; py, person-years.
aP = .001 for comparison with 18–22-year-olds.
bP = .03 for comparison with 18–22-year-olds.
cP = .002 for comparison with 18–22-year-olds.
dP ≤ .0001 for comparison with non-Hispanic white participants.
Rate and Frequency of Herpes Simplex Virus Infection in Healthy Young Women
| . | . | Rate per 100 py (No. of Cases) . | |||
|---|---|---|---|---|---|
| Infection Type . | No. . | All Infections . | Unrecognized Infection . | Genital Disease . | Oral Disease . |
| HSV 1 | |||||
| All subjects | 3438 | 2.5 (127) | 1.9 (94) | 0.5 (28) | 0.2 (9) |
| 18–22 y | 1928 | 3.2 (89) | 2.5 (71) | 0.5 (15) | 0.2 (5) |
| 23–26 y | 1063 | 1.6 (26)c | 1.0 (16)a | 0.5 (8) | 0.2 (3) |
| 27–30 y | 447 | 1.8 (12) | 1.0 (7)b | 0.7 (5) | 0.1 (1) |
| Non-Hispanic white | 2693 | 2.6 (104) | 1.9 (76) | 0.6 (24) | 0.2 (8) |
| Non-Hispanic black | 342 | 1.5 (7) | 1.1 (5) | 0.4 (2) | 0.0 (0) |
| Hispanic | 168 | 2.6 (6) | 1.7 (4) | 0.4 (1) | 0.4 (1) |
| Other | 235 | 3.0 (10) | 2.7 (9) | 0.3 (1) | 0.0 (0) |
| HSV 2 | |||||
| All subjects | 3438 | 1.1 (56) | 0.7 (35) | 0.4 (21) | |
| 18–22 y | 1928 | 1.3 (37) | 0.8 (22) | 0.5 (15) | |
| 23–26 y | 1063 | 0.8 (13) | 0.5 (8) | 0.3 (5) | |
| 27–30 y | 447 | 0.9 (6) | 0.7 (5) | 0.1 (1) | |
| Non-Hispanic white | 2693 | 0.8 (31) | 0.5 (21) | 0.2 (10) | |
| Non-Hispanic black | 342 | 4.4 (20)d | 2.6 (12)d | 1.7 (8)d | |
| Hispanic | 168 | 1.7 (4) | 0.8 (2) | 0.8 (2) | |
| Other | 235 | 0.3 (1) | 0.0 (0) | 0.3 (1) | |
| . | . | Rate per 100 py (No. of Cases) . | |||
|---|---|---|---|---|---|
| Infection Type . | No. . | All Infections . | Unrecognized Infection . | Genital Disease . | Oral Disease . |
| HSV 1 | |||||
| All subjects | 3438 | 2.5 (127) | 1.9 (94) | 0.5 (28) | 0.2 (9) |
| 18–22 y | 1928 | 3.2 (89) | 2.5 (71) | 0.5 (15) | 0.2 (5) |
| 23–26 y | 1063 | 1.6 (26)c | 1.0 (16)a | 0.5 (8) | 0.2 (3) |
| 27–30 y | 447 | 1.8 (12) | 1.0 (7)b | 0.7 (5) | 0.1 (1) |
| Non-Hispanic white | 2693 | 2.6 (104) | 1.9 (76) | 0.6 (24) | 0.2 (8) |
| Non-Hispanic black | 342 | 1.5 (7) | 1.1 (5) | 0.4 (2) | 0.0 (0) |
| Hispanic | 168 | 2.6 (6) | 1.7 (4) | 0.4 (1) | 0.4 (1) |
| Other | 235 | 3.0 (10) | 2.7 (9) | 0.3 (1) | 0.0 (0) |
| HSV 2 | |||||
| All subjects | 3438 | 1.1 (56) | 0.7 (35) | 0.4 (21) | |
| 18–22 y | 1928 | 1.3 (37) | 0.8 (22) | 0.5 (15) | |
| 23–26 y | 1063 | 0.8 (13) | 0.5 (8) | 0.3 (5) | |
| 27–30 y | 447 | 0.9 (6) | 0.7 (5) | 0.1 (1) | |
| Non-Hispanic white | 2693 | 0.8 (31) | 0.5 (21) | 0.2 (10) | |
| Non-Hispanic black | 342 | 4.4 (20)d | 2.6 (12)d | 1.7 (8)d | |
| Hispanic | 168 | 1.7 (4) | 0.8 (2) | 0.8 (2) | |
| Other | 235 | 0.3 (1) | 0.0 (0) | 0.3 (1) | |
Abbreviations: HSV, herpes simplex virus; py, person-years.
aP = .001 for comparison with 18–22-year-olds.
bP = .03 for comparison with 18–22-year-olds.
cP = .002 for comparison with 18–22-year-olds.
dP ≤ .0001 for comparison with non-Hispanic white participants.
Natural history of herpes simplex virus (HSV) infections in herpes simplex virus (HSV)–seronegative women aged 18–30 years. The analysis presented here includes the 3438 women who only received the control hepatitis A vaccine (Havrix) in the HERPEVAC trial, were not infected with HSV at baseline, and had at least 1 follow-up visit. Abbreviation: HSV, herpes simplex virus.
Genital HSV Disease
We compared the clinical manifestations of genital disease caused by HSV-1 (n = 28) to those caused by HSV-2 (n = 21) in the 49 women who developed genital symptoms and lesions (Table 2). Lesions were reported by 93% of participants with HSV-1 and 81% of participants with HSV-2 genital disease (P = .38). Similarly, lesions were documented by the clinician in 71% of participants with either HSV-1 or HSV-2 genital disease. The most frequent lesion types reported by participants were similar for HSV-1 and HSV-2: ulcers (HSV-1, 75%; HSV-2, 52%), vesicles (HSV-1, 64%; HSV-2, 48%), and papules (HSV-1, 61%; HSV-2, 57%). Approximately 50% of women with genital HSV disease had systemic symptoms, most commonly malaise, regardless of the HSV type (Table 2). Fever was noted in 18% of those infected with HSV-1 and 33% of those infected with HSV-2 (P = .32). Muscle aches were reported by 36% of participants with HSV-1 and 38% of participants with HSV-2 (P = 1.0). Additionally, local symptoms of genital HSV-1 or HSV-2 disease were similar, with about 90% reporting pain, burning, or itching, and about 50% reporting a vaginal discharge.
Local Manifestations of Primary Symptomatic Genital and Oral Herpes Disease
| . | Genital Herpes . | Oral Herpes . | ||||
|---|---|---|---|---|---|---|
| . | HSV-1 (n = 28) . | HSV-2 (n = 21) . | HSV-1 (n = 9) . | |||
| . | Clinician Report . | Subject Diary Card . | Clinician Report . | Subject Diary Card . | Clinician Report . | Subject Diary Card . |
| Lesion type, No. (%) | ||||||
| Any lesions | 20 (71.4) | 26 (92.9) | 15 (71.4) | 17 (81) | 5 (55.6) | 9 (100) |
| Papules | 8 (28.6) | 17 (60.7) | 6 (28.6) | 12 (57.1) | 1 (11.1) | 6 (66.7) |
| Vesicles | 12 (42.9) | 18 (64.3) | 7 (33.3) | 10 (47.6) | 3 (33.3) | 4 (44.4) |
| Ulcers | 14 (50) | 21 (75) | 10 (47.6) | 11 (52.4) | 2 (22.2) | 3 (33.3) |
| Crusts | 6 (21.4) | 11 (39.3) | 2 (9.5) | 5 (23.8) | 1 (11.1) | 4 (44.4) |
| Fissuresa | 3 (10.7) | 8 (28.6) | 1 (4.8) | 1 (11.1) | 3 (33.3) | |
| Lesion location,b No. (%) | ||||||
| Orolabial | 1 (4.8) | 9 (100) | ||||
| Genital | 25 (89.3) | 20 (95.2) | ||||
| Buttocks | 1 (3.6) | |||||
| Rectal | 4 (14.3) | 1 (4.8) | ||||
| Otherc | 1 (3.6) | 2 (9.5) | ||||
| Multiple sites | 4 (14.3) | 2 (9.5) | ||||
| Local symptoms,d No. (%) | ||||||
| Any local symptoms | 26 (92.9) | 18 (85.7) | 9 (100) | |||
| Pain/burning/itching | 26 (92.9) | 17 (81) | 9 (100) | |||
| Pain with urination | 20 (71.4) | 13 (61.9) | ||||
| Redness | 22 (78.6) | 15 (71.4) | 4 (44.4) | |||
| Vaginal discharge | 12 (42.9) | 13 (61.9) | ||||
| Swelling | 17 (60.7) | 11 (52.4) | 2 (22.2) | |||
| Systemic symptoms, No. (%) | ||||||
| Any systemic symptoms | 16 (57.1) | 9 (42.9) | 5 (55.6) | |||
| Headache | 9 (32.1) | 7 (33.3) | 4 (44.4) | |||
| Malaise | 14 (50) | 8 (38.1) | 4 (44.4) | |||
| Muscle aches | 10 (35.7) | 8 (38.1) | 2 (22.2) | |||
| Fever | 5 (17.9) | 7 (33.3) | 3 (33.3) | |||
| Duration, d, median (min, max) | ||||||
| Any lesions/symptoms | 12.0 (5, 62) | 10.0 (4, 19) | 9.0 (3,326) | |||
| Lesions | 10.0 (4, 51) | 8.0 (4, 14) | 7.0 (3, 326) | |||
| Local symptoms | 10.5 (1, 62) | 10.0 (3, 18) | 5.0 (1, 326) | |||
| Systemic symptoms | 7.5 (1, 18) | 6.0 (2, 17) | 4.0 (3, 13) | |||
| . | Genital Herpes . | Oral Herpes . | ||||
|---|---|---|---|---|---|---|
| . | HSV-1 (n = 28) . | HSV-2 (n = 21) . | HSV-1 (n = 9) . | |||
| . | Clinician Report . | Subject Diary Card . | Clinician Report . | Subject Diary Card . | Clinician Report . | Subject Diary Card . |
| Lesion type, No. (%) | ||||||
| Any lesions | 20 (71.4) | 26 (92.9) | 15 (71.4) | 17 (81) | 5 (55.6) | 9 (100) |
| Papules | 8 (28.6) | 17 (60.7) | 6 (28.6) | 12 (57.1) | 1 (11.1) | 6 (66.7) |
| Vesicles | 12 (42.9) | 18 (64.3) | 7 (33.3) | 10 (47.6) | 3 (33.3) | 4 (44.4) |
| Ulcers | 14 (50) | 21 (75) | 10 (47.6) | 11 (52.4) | 2 (22.2) | 3 (33.3) |
| Crusts | 6 (21.4) | 11 (39.3) | 2 (9.5) | 5 (23.8) | 1 (11.1) | 4 (44.4) |
| Fissuresa | 3 (10.7) | 8 (28.6) | 1 (4.8) | 1 (11.1) | 3 (33.3) | |
| Lesion location,b No. (%) | ||||||
| Orolabial | 1 (4.8) | 9 (100) | ||||
| Genital | 25 (89.3) | 20 (95.2) | ||||
| Buttocks | 1 (3.6) | |||||
| Rectal | 4 (14.3) | 1 (4.8) | ||||
| Otherc | 1 (3.6) | 2 (9.5) | ||||
| Multiple sites | 4 (14.3) | 2 (9.5) | ||||
| Local symptoms,d No. (%) | ||||||
| Any local symptoms | 26 (92.9) | 18 (85.7) | 9 (100) | |||
| Pain/burning/itching | 26 (92.9) | 17 (81) | 9 (100) | |||
| Pain with urination | 20 (71.4) | 13 (61.9) | ||||
| Redness | 22 (78.6) | 15 (71.4) | 4 (44.4) | |||
| Vaginal discharge | 12 (42.9) | 13 (61.9) | ||||
| Swelling | 17 (60.7) | 11 (52.4) | 2 (22.2) | |||
| Systemic symptoms, No. (%) | ||||||
| Any systemic symptoms | 16 (57.1) | 9 (42.9) | 5 (55.6) | |||
| Headache | 9 (32.1) | 7 (33.3) | 4 (44.4) | |||
| Malaise | 14 (50) | 8 (38.1) | 4 (44.4) | |||
| Muscle aches | 10 (35.7) | 8 (38.1) | 2 (22.2) | |||
| Fever | 5 (17.9) | 7 (33.3) | 3 (33.3) | |||
| Duration, d, median (min, max) | ||||||
| Any lesions/symptoms | 12.0 (5, 62) | 10.0 (4, 19) | 9.0 (3,326) | |||
| Lesions | 10.0 (4, 51) | 8.0 (4, 14) | 7.0 (3, 326) | |||
| Local symptoms | 10.5 (1, 62) | 10.0 (3, 18) | 5.0 (1, 326) | |||
| Systemic symptoms | 7.5 (1, 18) | 6.0 (2, 17) | 4.0 (3, 13) | |||
Abbreviation: HSV, herpex simplex virus.
aP = .0003 for comparison between HSV-1 and HSV-2 for symptoms collected on subject diary card.
b A subject may have had >1 location of lesions recorded.
c “Other” locations specified by clinician for 3 subjects included (1) internal genital, (2) cervix and mons pubis, (3) cervicovaginal.
d Local and systemic symptoms were only recorded on the subjects’ diary cards.
Local Manifestations of Primary Symptomatic Genital and Oral Herpes Disease
| . | Genital Herpes . | Oral Herpes . | ||||
|---|---|---|---|---|---|---|
| . | HSV-1 (n = 28) . | HSV-2 (n = 21) . | HSV-1 (n = 9) . | |||
| . | Clinician Report . | Subject Diary Card . | Clinician Report . | Subject Diary Card . | Clinician Report . | Subject Diary Card . |
| Lesion type, No. (%) | ||||||
| Any lesions | 20 (71.4) | 26 (92.9) | 15 (71.4) | 17 (81) | 5 (55.6) | 9 (100) |
| Papules | 8 (28.6) | 17 (60.7) | 6 (28.6) | 12 (57.1) | 1 (11.1) | 6 (66.7) |
| Vesicles | 12 (42.9) | 18 (64.3) | 7 (33.3) | 10 (47.6) | 3 (33.3) | 4 (44.4) |
| Ulcers | 14 (50) | 21 (75) | 10 (47.6) | 11 (52.4) | 2 (22.2) | 3 (33.3) |
| Crusts | 6 (21.4) | 11 (39.3) | 2 (9.5) | 5 (23.8) | 1 (11.1) | 4 (44.4) |
| Fissuresa | 3 (10.7) | 8 (28.6) | 1 (4.8) | 1 (11.1) | 3 (33.3) | |
| Lesion location,b No. (%) | ||||||
| Orolabial | 1 (4.8) | 9 (100) | ||||
| Genital | 25 (89.3) | 20 (95.2) | ||||
| Buttocks | 1 (3.6) | |||||
| Rectal | 4 (14.3) | 1 (4.8) | ||||
| Otherc | 1 (3.6) | 2 (9.5) | ||||
| Multiple sites | 4 (14.3) | 2 (9.5) | ||||
| Local symptoms,d No. (%) | ||||||
| Any local symptoms | 26 (92.9) | 18 (85.7) | 9 (100) | |||
| Pain/burning/itching | 26 (92.9) | 17 (81) | 9 (100) | |||
| Pain with urination | 20 (71.4) | 13 (61.9) | ||||
| Redness | 22 (78.6) | 15 (71.4) | 4 (44.4) | |||
| Vaginal discharge | 12 (42.9) | 13 (61.9) | ||||
| Swelling | 17 (60.7) | 11 (52.4) | 2 (22.2) | |||
| Systemic symptoms, No. (%) | ||||||
| Any systemic symptoms | 16 (57.1) | 9 (42.9) | 5 (55.6) | |||
| Headache | 9 (32.1) | 7 (33.3) | 4 (44.4) | |||
| Malaise | 14 (50) | 8 (38.1) | 4 (44.4) | |||
| Muscle aches | 10 (35.7) | 8 (38.1) | 2 (22.2) | |||
| Fever | 5 (17.9) | 7 (33.3) | 3 (33.3) | |||
| Duration, d, median (min, max) | ||||||
| Any lesions/symptoms | 12.0 (5, 62) | 10.0 (4, 19) | 9.0 (3,326) | |||
| Lesions | 10.0 (4, 51) | 8.0 (4, 14) | 7.0 (3, 326) | |||
| Local symptoms | 10.5 (1, 62) | 10.0 (3, 18) | 5.0 (1, 326) | |||
| Systemic symptoms | 7.5 (1, 18) | 6.0 (2, 17) | 4.0 (3, 13) | |||
| . | Genital Herpes . | Oral Herpes . | ||||
|---|---|---|---|---|---|---|
| . | HSV-1 (n = 28) . | HSV-2 (n = 21) . | HSV-1 (n = 9) . | |||
| . | Clinician Report . | Subject Diary Card . | Clinician Report . | Subject Diary Card . | Clinician Report . | Subject Diary Card . |
| Lesion type, No. (%) | ||||||
| Any lesions | 20 (71.4) | 26 (92.9) | 15 (71.4) | 17 (81) | 5 (55.6) | 9 (100) |
| Papules | 8 (28.6) | 17 (60.7) | 6 (28.6) | 12 (57.1) | 1 (11.1) | 6 (66.7) |
| Vesicles | 12 (42.9) | 18 (64.3) | 7 (33.3) | 10 (47.6) | 3 (33.3) | 4 (44.4) |
| Ulcers | 14 (50) | 21 (75) | 10 (47.6) | 11 (52.4) | 2 (22.2) | 3 (33.3) |
| Crusts | 6 (21.4) | 11 (39.3) | 2 (9.5) | 5 (23.8) | 1 (11.1) | 4 (44.4) |
| Fissuresa | 3 (10.7) | 8 (28.6) | 1 (4.8) | 1 (11.1) | 3 (33.3) | |
| Lesion location,b No. (%) | ||||||
| Orolabial | 1 (4.8) | 9 (100) | ||||
| Genital | 25 (89.3) | 20 (95.2) | ||||
| Buttocks | 1 (3.6) | |||||
| Rectal | 4 (14.3) | 1 (4.8) | ||||
| Otherc | 1 (3.6) | 2 (9.5) | ||||
| Multiple sites | 4 (14.3) | 2 (9.5) | ||||
| Local symptoms,d No. (%) | ||||||
| Any local symptoms | 26 (92.9) | 18 (85.7) | 9 (100) | |||
| Pain/burning/itching | 26 (92.9) | 17 (81) | 9 (100) | |||
| Pain with urination | 20 (71.4) | 13 (61.9) | ||||
| Redness | 22 (78.6) | 15 (71.4) | 4 (44.4) | |||
| Vaginal discharge | 12 (42.9) | 13 (61.9) | ||||
| Swelling | 17 (60.7) | 11 (52.4) | 2 (22.2) | |||
| Systemic symptoms, No. (%) | ||||||
| Any systemic symptoms | 16 (57.1) | 9 (42.9) | 5 (55.6) | |||
| Headache | 9 (32.1) | 7 (33.3) | 4 (44.4) | |||
| Malaise | 14 (50) | 8 (38.1) | 4 (44.4) | |||
| Muscle aches | 10 (35.7) | 8 (38.1) | 2 (22.2) | |||
| Fever | 5 (17.9) | 7 (33.3) | 3 (33.3) | |||
| Duration, d, median (min, max) | ||||||
| Any lesions/symptoms | 12.0 (5, 62) | 10.0 (4, 19) | 9.0 (3,326) | |||
| Lesions | 10.0 (4, 51) | 8.0 (4, 14) | 7.0 (3, 326) | |||
| Local symptoms | 10.5 (1, 62) | 10.0 (3, 18) | 5.0 (1, 326) | |||
| Systemic symptoms | 7.5 (1, 18) | 6.0 (2, 17) | 4.0 (3, 13) | |||
Abbreviation: HSV, herpex simplex virus.
aP = .0003 for comparison between HSV-1 and HSV-2 for symptoms collected on subject diary card.
b A subject may have had >1 location of lesions recorded.
c “Other” locations specified by clinician for 3 subjects included (1) internal genital, (2) cervix and mons pubis, (3) cervicovaginal.
d Local and systemic symptoms were only recorded on the subjects’ diary cards.
Oral HSV Disease
All oral HSV disease (n = 9) was caused by HSV-1. Systemic symptoms were reported by 56% of the participants. Local symptoms included pain/burning/itching (100%), redness (44%), and swelling (22%).
Accuracy of Clinical Assessment for Genital HSV Disease
When clinicians assessed the participants presenting for possible genital herpes as clinically confirmed herpes, their assessments were validated by HSV culture and/or serological test results 83% of the time (Table 3). When their assessment was that genital herpes was possible, 30% were validated. No cases that the investigator felt were not genital herpes were confirmed as genital herpes by laboratory testing.
Accuracy of Clinician Assessment
| . | Confirmed by Culture and/or Serology, No. (%) . | |
|---|---|---|
| Clinician Assessment . | No . | Yes . |
| Clinically confirmed herpes | 4 (17) | 19 (83) |
| Possibly herpes | 28 (70) | 12 (30) |
| Unlikely to be herpes | 103 (100) | 0 |
| . | Confirmed by Culture and/or Serology, No. (%) . | |
|---|---|---|
| Clinician Assessment . | No . | Yes . |
| Clinically confirmed herpes | 4 (17) | 19 (83) |
| Possibly herpes | 28 (70) | 12 (30) |
| Unlikely to be herpes | 103 (100) | 0 |
Thirteen subjects with missing investigator assessment or clinical exam date were excluded.
Accuracy of Clinician Assessment
| . | Confirmed by Culture and/or Serology, No. (%) . | |
|---|---|---|
| Clinician Assessment . | No . | Yes . |
| Clinically confirmed herpes | 4 (17) | 19 (83) |
| Possibly herpes | 28 (70) | 12 (30) |
| Unlikely to be herpes | 103 (100) | 0 |
| . | Confirmed by Culture and/or Serology, No. (%) . | |
|---|---|---|
| Clinician Assessment . | No . | Yes . |
| Clinically confirmed herpes | 4 (17) | 19 (83) |
| Possibly herpes | 28 (70) | 12 (30) |
| Unlikely to be herpes | 103 (100) | 0 |
Thirteen subjects with missing investigator assessment or clinical exam date were excluded.
Univariate logistic regression analysis of participants reporting signs and symptoms of genital herpes demonstrated significant associations between the diagnosis of genital HSV disease and the presence of vesicles, ulcers, pain, painful urination, redness, swelling, malaise, and muscle aches. These 8 variables were considered for inclusion in a multivariate logistic regression model. A forward selection algorithm resulted in a model with 4 predictors (in order of selection): ulcers, vesicles, painful urination, and pain, which were used to define 8 classification rules: classify with HSV disease if woman has (1) ulcers; (2) ulcers or vesicles; (3) ulcers or vesicles or painful urination; (4) ulcers or vesicles or painful urination or pain; (5) at least 1 of the 4 signs/symptoms; (6) at least 2 of the 4 signs/symptoms; (7) at least 3 of the 4 signs/symptoms; (8) all 4 of the 4 signs/symptoms. The predictive ability of each classification rule is summarized in Table 4. Specificity, positive predictive value, and accuracy for the diagnosis of genital herpes were all highest when ulcers were present.
Signs and Symptoms Predictive of Laboratory-Confirmed Genital Herpes Disease
| Diagnostic Rule . | Sensitivity . | Specificity . | PPV . | NPV . | Accuracy . |
|---|---|---|---|---|---|
| Rule based on occurrence of specific signs or symptoms | |||||
| Ulcers | 0.63 | 0.89 | 0.69 | 0.86 | 0.82 |
| Ulcers or vesicles | 0.76 | 0.81 | 0.60 | 0.90 | 0.79 |
| Ulcers or vesicles or pain | 0.87 | 0.68 | 0.51 | 0.93 | 0.73 |
| Ulcers or vesicles or painful urination or pain | 0.98 | 0.26 | 0.34 | 0.97 | 0.46 |
| Rule based on No. of the following signs/symptoms: ulcers, vesicles, painful urination, or pain | |||||
| At least 1 of 4 | 0.98 | 0.26 | 0.34 | 0.97 | 0.46 |
| At least 2 of 4 | 0.85 | 0.73 | 0.55 | 0.93 | 0.76 |
| At least 3 of 4 | 0.59 | 0.95 | 0.82 | 0.86 | 0.85 |
| All 4 | 0.39 | 1.00 | 1.00 | 0.81 | 0.83 |
| Diagnostic Rule . | Sensitivity . | Specificity . | PPV . | NPV . | Accuracy . |
|---|---|---|---|---|---|
| Rule based on occurrence of specific signs or symptoms | |||||
| Ulcers | 0.63 | 0.89 | 0.69 | 0.86 | 0.82 |
| Ulcers or vesicles | 0.76 | 0.81 | 0.60 | 0.90 | 0.79 |
| Ulcers or vesicles or pain | 0.87 | 0.68 | 0.51 | 0.93 | 0.73 |
| Ulcers or vesicles or painful urination or pain | 0.98 | 0.26 | 0.34 | 0.97 | 0.46 |
| Rule based on No. of the following signs/symptoms: ulcers, vesicles, painful urination, or pain | |||||
| At least 1 of 4 | 0.98 | 0.26 | 0.34 | 0.97 | 0.46 |
| At least 2 of 4 | 0.85 | 0.73 | 0.55 | 0.93 | 0.76 |
| At least 3 of 4 | 0.59 | 0.95 | 0.82 | 0.86 | 0.85 |
| All 4 | 0.39 | 1.00 | 1.00 | 0.81 | 0.83 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
Signs and Symptoms Predictive of Laboratory-Confirmed Genital Herpes Disease
| Diagnostic Rule . | Sensitivity . | Specificity . | PPV . | NPV . | Accuracy . |
|---|---|---|---|---|---|
| Rule based on occurrence of specific signs or symptoms | |||||
| Ulcers | 0.63 | 0.89 | 0.69 | 0.86 | 0.82 |
| Ulcers or vesicles | 0.76 | 0.81 | 0.60 | 0.90 | 0.79 |
| Ulcers or vesicles or pain | 0.87 | 0.68 | 0.51 | 0.93 | 0.73 |
| Ulcers or vesicles or painful urination or pain | 0.98 | 0.26 | 0.34 | 0.97 | 0.46 |
| Rule based on No. of the following signs/symptoms: ulcers, vesicles, painful urination, or pain | |||||
| At least 1 of 4 | 0.98 | 0.26 | 0.34 | 0.97 | 0.46 |
| At least 2 of 4 | 0.85 | 0.73 | 0.55 | 0.93 | 0.76 |
| At least 3 of 4 | 0.59 | 0.95 | 0.82 | 0.86 | 0.85 |
| All 4 | 0.39 | 1.00 | 1.00 | 0.81 | 0.83 |
| Diagnostic Rule . | Sensitivity . | Specificity . | PPV . | NPV . | Accuracy . |
|---|---|---|---|---|---|
| Rule based on occurrence of specific signs or symptoms | |||||
| Ulcers | 0.63 | 0.89 | 0.69 | 0.86 | 0.82 |
| Ulcers or vesicles | 0.76 | 0.81 | 0.60 | 0.90 | 0.79 |
| Ulcers or vesicles or pain | 0.87 | 0.68 | 0.51 | 0.93 | 0.73 |
| Ulcers or vesicles or painful urination or pain | 0.98 | 0.26 | 0.34 | 0.97 | 0.46 |
| Rule based on No. of the following signs/symptoms: ulcers, vesicles, painful urination, or pain | |||||
| At least 1 of 4 | 0.98 | 0.26 | 0.34 | 0.97 | 0.46 |
| At least 2 of 4 | 0.85 | 0.73 | 0.55 | 0.93 | 0.76 |
| At least 3 of 4 | 0.59 | 0.95 | 0.82 | 0.86 | 0.85 |
| All 4 | 0.39 | 1.00 | 1.00 | 0.81 | 0.83 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
HSV Antibody Response
When the 28 participants with HSV-1 genital disease (23 culture confirmed) and the 21 participants with HSV-2 genital disease (16 culture confirmed) were evaluated by WB, all but 3 women with HSV-1 infection seroconverted after developing genital herpes. Of these, 1 did not return for follow-up and 2 had not developed antibodies at 8 and 13 months, after culture-confirmed HSV-1 genital infection. Of the 5 participants who developed oral disease without genital disease, 3 had disease confirmed by culture and all seroconverted to HSV-1. Of particular interest, 6 participants (1 genital HSV-1, 4 genital HSV-2, and 1 oral HSV-1) seroconverted prior to developing recognized symptoms of herpes disease (data not shown). Their first recognized symptoms occurred from 176 to 319 days after they were first infected.
During the study, 32 participants developed an indeterminate WB (14 for HSV-1, 13 for HSV-2, and 5 for both HSV-1 and HSV-2). A designation of indeterminate is assigned when the test does not meet the definition for a positive but has some evidence indicating the presence of HSV antibody. Of these participants, 25 later developed a positive WB response (average, 184 days after the first indeterminate result [range, 30–434 days]). Seven participants with an indeterminate WB did not develop a positive WB during study follow-up, 3 did not have any further samples tested after the indeterminate result was obtained, and 4 remained indeterminate throughout study follow-up (range, 23–421 days).
DISCUSSION
This is the largest prospective study of HSV acquisition in HSV-seronegative women ever performed. It confirms and extends several observations of primary HSV infections. The rate of infection for HSV-1 (2.5 per 100 py) was more than twice that for HSV-2 (1.1 per 100 py) in young women. This is quite different from the rate of 1.0 case per 100 py for HSV-1 and 6.8 per 100 py for HSV-2 in women in another prospective study evaluating participants in an HSV vaccine study conducted from 1993 to 1995 [8]. However, that study was enriched for women with a high risk of exposure to HSV-2 (HSV-discordant couples and sexually transmitted infection [STI] clinic attendees). Further, women with and without previous HSV-1 infections were included and only women with ≥4 sexual partners in the prior year prior were enrolled. In a previous prospective study of women recruited from STI clinics from 1992 to 1995, the HSV-2 infection rate was also considerably higher, 20.5 per 100 py [9], whereas in a study of young adolescents conducted in early 2000, the rate was 4.4 and 3.2 per 100 py for HSV-2 and HSV-1, respectively [10]. The higher HSV-2 rate of infection in this study may reflect the predominance of non-Hispanic black participants in this trial.
In the young adult women studied here, clinically recognized HSV-1 infections presented 3 times more commonly as genital than oral disease. Thus, while HSV-1 acquired in childhood occurs as oral infections, in young adults the majority may be acquired as genital infection. This finding must be tempered by the fact that while participants were instructed to report any oral or genital HSV disease, the emphasis for the study was genital disease. Furthermore, the majority (74%) of HSV-1 infections did not produce recognizable disease and thus the site of infection is unknown.
Infection rates for HSV-1 and HSV-2 differed markedly between racial groups. HSV-2 infections accounted for 74% of HSV infections in non-Hispanic blacks (the rate of infection was 2.6 times higher than in Hispanics and 5.5 times higher than in non-Hispanic whites). In contrast, the rate of HSV-1 infection was 1.7 times higher in non-Hispanic whites than in non-Hispanic blacks, and it was identical in Hispanics and non-Hispanic whites. In a previous study, [8] the HSV-2 acquisition rate was similarly nearly double for non-white women (11.2 per 100 py) compared with white women (5.8 per 100 py). This suggests that there are differences in the prevalence of HSV types in the source partners of the study participants as documented in large sero-surveys [11, 12]. Alternatively, the variability in type-specific HSV rates of infection may be in part attributable to race-based differences in sexual practices or other behaviors.
The rates of infection and the development of recognized HSV disease also differed by age. Younger participants were more likely to acquire HSV-1 infections compared with older participants and less likely to develop recognized disease. Differences in HSV type may reflect differences in sexual practices by age, with younger participants more likely to engage in oral than vaginal sex [13]. Differences in the development of recognized signs and symptoms of HSV disease may reflect a maturing ability to recognize changes in sexual health or a real difference in the development of the signs and symptoms.
As noted in previous studies, [8, 13–15] most primary infections with HSV do not produce recognized signs or symptoms. In the study reported here, 74% of HSV-1 and 63% of HSV-2 infections did not produce participant-recognized disease (or at least did not bring them to the study clinics). Thus, despite educational efforts, most participants were unaware of their infections and would therefore not undertake efforts to prevent transmission.
The manifestations of HSV-1 were compared to HSV-2 and, as previously noted [16], the infections produced indistinguishable disease.
As reported previously [17, 18], the first clinical presentation of genital HSV can occur long after the primary infection. In the study reported here, 6 participants were infected and developed HSV antibody responses 176–319 days before developing the first oral (n = 1) or genital (n = 5) symptoms of HSV. In a previous study, 8% of participants developed their first clinical episode of genital HSV-2 after developing HSV-2 antibodies [8].
Western blots are often obtained to determine if a patient has been infected with HSV. Physicians may receive a report of indeterminate results either because the infection is recent or for other unexplained reasons. Of the 32 WBs reported as indeterminate, 25, became positive and 7 remained indeterminate Thus, the majority were infected with HSV.
Finally, it is difficult to distinguish genital HSV infections from other diseases that cause similar signs and symptoms by clinical exam, and thus culture (or polymerase chain reaction) and/or serology is recommended as an aid to the clinician. [14, 15, 19]. We developed a multivariate logistic regression model using a forward selection algorithm of 8 signs or symptoms that were associated with genital HSV by univariate analysis. This resulted in a model with 4 predictors, which were used to define rules for identifying genital HSV infections. Use of only 1 of these signs/symptoms to identify genital HSV infections had the highest sensitivity (98%), whereas the rule that required all 4 to be present had the best specificity (100%) and accuracy (83%).
There are several factors that might influence our findings and their generalizability. The population evaluated was a diverse group of women, 18–30 years of age. However, they represent a self-selected sample that volunteered to be in a study of a herpes vaccine and therefore could conceivable be more at risk for acquiring an HSV infection. Thus, it is possible our estimates of rates of infection could be somewhat higher than the general population of young women.
In summary, this is the largest prospective study of documented primary HSV infections in which the identification of symptomatic vs unrecognized primary infections could be categorized with a high degree of certainty. We found that, overall, the rate of infection for HSV-1 was higher than for HSV-2, but that there were significant age and racial differences. Infections by either type were most often not recognized by the participants, despite the efforts to educate them regarding possible signs and symptoms of HSV disease. These findings have important implications for the design and implementation of treatment and prevention strategies.
Notes
Author contributions. D. I. B. and R. B. B. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (N01-AI-45250) and GlaxoSmithKline.
Potential conflicts of interest. D. I. B. has received lecture fees and royalties from GlaxoSmithKline. R. B. B. has served as a board member of Vivaldi Biosciences, has received consulting fees from GlaxoSmithKline, has received consulting fees and lecture fees from MedImmune, and has received lecture fees from Merck. G. D. is an employee of and has received stock and travel, accommodations, and meeting expenses from GlaxoSmithKline and royalties from Pfizer. T. C. H. is an employee of GlaxoSmithKline and has received travel, accommodations, meeting expenses, and stock equity from GlaxoSmithKline. M. J. L. has received consulting fees and grants from GlaxoSmithKline. A. W. has received consulting fees from AiCuris, Agenus, and ViruLite, and grant support from Genocea, Gilead, GlaxoSmithKline, and the Washington Vaccine Alliance. E. W. H. has received consulting fees from Cempra, grants from Becton Dickinson and GlaxoSmithKline, honoraria from Becton Dickinson, and royalties from McGraw-Hill. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments