Article Text
Abstract
Objectives Systematic screening for Chlamydia trachomatis by individual invitation can be optimised by filtering participants on risk profile, excluding people at no or low risk. The authors investigated this technique in a large-scale chlamydia screening programme in the Netherlands in one rural region where relatively low prevalence was expected (<2%).
Methods Invitees were alerted by personal letter to log in to http://www.chlamydiatest.nl and fill in an 8-item questionnaire. Only invitees with sufficient score could proceed to request a test kit. The authors investigated the effect of selection on participation, positivity and acceptability in three screening rounds and on the number needed to invite and the number needed to screen.
Results The selection led to exclusion of 36% of potential participants and a positivity rate of 4.8% among participants, achieving similar number needed to screen values in the rural and urban areas. Higher scores were clearly related to higher positivity rates. Persons who were excluded from participation did not have a lower response in the next round. The acceptability study revealed disappointment about exclusion of 30% of excluded participants but most approved of the screening set-up.
Conclusions Systematic selection of screening participants by risk score is feasible and successful in realising higher positivity rates. A somewhat stricter selection could be applied in the rural and urban areas of the screening programme. Multiple-item selection with a cut-off total score may work better than, more commonly used, selection by single criteria, especially in low-risk populations. Acceptability of selection is high but could still be improved by better communication on expectations.
- Chlamydia trachomatis
- screening
- risk score
- selection tool
- participation
- positivity
- acceptability
- epidemiology (general)
- surveillance
- general practice
- clinical STI care
- syndromic management
- HIV
- epidemiology (molecular)
- chlamydia infection
Statistics from Altmetric.com
- Chlamydia trachomatis
- screening
- risk score
- selection tool
- participation
- positivity
- acceptability
- epidemiology (general)
- surveillance
- general practice
- clinical STI care
- syndromic management
- HIV
- epidemiology (molecular)
- chlamydia infection
Introduction
Chlamydia trachomatis (Ct) infection is the most prevalent treatable sexually transmitted infection (STI). It often affects young heterosexual people, while most other STIs are more common in other risk groups, such as homosexual men.1 2 Repeated infections occur due to no or limited development of immunity.3 Cases often stay asymptomatic, while in the long term, infections can cause serious adverse events, such as pelvic inflammatory disease, tubal pathology and infertility.4 To detect asymptomatic cases and prevent these adverse events, screening is the intervention of choice, although good evidence to support the effectiveness of screening is still lacking.5
Systematic screening by inviting the whole target population ensures that everyone is reached but has the disadvantage that also people at no/low risk for Ct will be tested, which might make the screening programme less cost-effective than screening designed to attract people with actual risk behaviour. Selective systematic screening can overcome this issue. A self-selection tool for potential participants in screening has not been applied before in a systematic Ct screening programme; the unique set-up with a short scoring questionnaire, as we present here, is especially practical with an internet-based program.
In 2008, a Chlamydia Screening Implementation (CSI) was rolled out in three areas of the Netherlands: two large cities, Amsterdam and Rotterdam, and one rural area in South Limburg.6 7 In the cities, all sexually active persons in the target group (men and women aged 16–29 years) were invited to participate without further selection. In the less urbanised region of South Limburg (‘Parkstad’), the expected Ct prevalence was lower than in the big cities, and therefore, in line with earlier recommendations,8 a selective systematic screening set-up was implemented using a selection risk score modelled on data from the pilot Ct.9
Here, we assess the effect of the risk score selection on participation and positivity in CSI and compare the effectiveness of screening expressed as the ‘number needed to invite’ (NNI) and the ‘number needed to screen’ (NNS) to find one Ct-positive case10 11 in South Limburg (selection score) versus Amsterdam and Rotterdam (universal screening). If selection is effective, it reduces the number of people getting tested, while it does hardly or not affect the number of Ct cases found. Hence, the NNI would remain high, while the NNS is reduced. The impact of further selection on the effectiveness of screening is discussed.
Methods
Screening and selection through risk score
The screening programme and evaluation were approved by the Medical Ethics Committee of the VU medical center in Amsterdam (METC number 2007/239). The target population invited for screening consisted of all persons from 16 to 29 years old registered in the municipal register. The screening was implemented in a stepped wedge design, that is, a roll-out of invitations cluster by cluster (town-area or village) covering the target population in the course of 1 year; in South Limburg, one-third of the target population was progressively added into the intervention per year in a risk stratified cluster-randomised order; in Amsterdam and Rotterdam, a control group of one-sixth of the population was invited only from the second round onwards. Three risk levels defined stratification of clusters: level of urbanisation (in Limburg) or proportion non-Dutch (cities), proportion of people 16–29 years old and proportion with low income per cluster (for more details, see van den Broek et al7).
All 16–29-year-old persons registered in selected clusters of 10 participating municipalities in Parkstad, South Limburg, received an invitation letter for the chlamydia screening by post. To participate, they were requested to log in to the website http://www.chlamydiatest.nl with their personal log in code provided in the letter. Online, they were first presented a questionnaire of eight questions. For each question, they could score points; for some issues, different points were assigned per answer for men and women:
Age 15–19 years (1 point).
Place of residence (medium urbanised (2 points) and highly urbanised (3 points).
Medium/lower level of education (2 points).
Antillean or Surinamese ethnicity (2 points).
Recent blood loss after sexual contact (women 1 point); Recent increased urge to urinate (men 2 points).
No condom used at last sexual intercourse (1 point).
Number of lifetime sex partners (2–5 men (2 points), women (3 points); 6 or more men (3 points), women (5 points).
New sexual partner in the last 6 months (1 point).
This prediction rule6 was developed in pilot Ct. In CSI, the cut-off point was again carefully considered. In first instance, a cut-off of 6 was chosen with the expectation; based on the prior study that by selection of all sexually active participants with a sum score of 6 or higher, the number to be screened would be reduced to 62%, while 7% of the Ct cases would then be missed (sensitivity 93%), assuming a prevalence of 3.5%. In 2008, anyone with a final score of 6 or more was advised to request a Ct test kit, while low scorers were unable to proceed to request a test kit at the website; the reason of low risk was explained. In 2009, however, the cut-off score was lowered to 5 because a relatively large group was excluded in the previous round. For the purpose of valid comparison, we present the main data based on a cut-off score of 6 for each round.
In Amsterdam and Rotterdam, no risk score questionnaire was used; screening was intended for all sexually active persons. All 16–29-year-old residents logging on to the website (assumed sexually active) were able to request a test kit. The majority of participants voluntarily answered an online questionnaire.
Analyses
We calculated the proportions excluded from participation by the selection process in South Limburg, the participation among those qualifying to participate and the positivity rate in this group. The relation between individual selection score items and participation and positivity was investigated by scoring profiles and logistic regression. We compared NNI (number invited/number positive) and NNS (number participated/number positive) between the three regions in CSI and in subgroups (gender, age groups and community risk level) with multinomial regression analysis. To estimate an optimal cut-off score for Limburg, we also calculated NNI and NNS and percentage of Ct-positives missed when a higher risk score would have been applied to select participants. We used PASW Statistics (SPSS) V.18 (IBM Corporation).
A small survey (n=200) was held among people who were excluded from participation to assess to what extent the fact that people were excluded from the screening caused problems or negative opinions on the screening and their willingness to be tested in the future (see also Op de Coul et al12).
Results
Selection, participation and positivity rates
The initial response rate in Limburg, that is, the percentage of persons who filled-in the online questionnaire was 22% in 2008, decreasing to 15% in 2009 and 11% in round 3 (tables 1 and 2).
Participation and selection in three screening rounds in South Limburg
Total number invited, respondents, participants and Ct-positives with estimated NNI and NNS per chlamydia case (Ct), per region and per round in the Chlamydia Screening Implementation Programme, 2008–2010
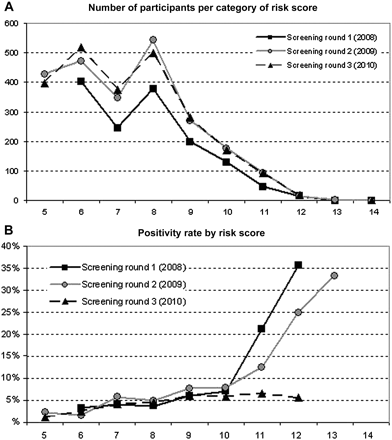
The number of points scored in the risk selection questionnaire varied from 0 to 14; most persons scored 6–8 points (figure 1A). Women scored on average higher than men (6.6 with 95% CI 5.7 to 5.9 vs 5.7 with 95% CI 6.5 to 6.7, p<0.001), partly due to the fact that women could score a maximum of 16 points versus 15 points in men. Overall, scores were significantly lower in round 1 than in rounds 2 and 3 (p<0.001, see mean scores in table 1). Most commonly, points were scored for urban residence, medium/lower education level, not using a condom at last sex contact and number of lifetime sex partners. The most influential determinants for actual participation were the same four as well as physical complaints (blood loss after intercourse or increased urination urge), while positivity related mostly to having started a new relationship in the last 6 months and number of lifetime partners.
Number of participants (A) and percentage testing positive for chlamydia (B) by risk score in Chlamydia Screening Implementation, South Limburg, 2008–2010.
With the overall initial response rate of 14.5% over three rounds and 63.6% selection by risk score, 9.2% of all invitees were actually able to participate. The majority of these (96%) requested a test kit, but about one in five (19.5%) did not return a sample to the laboratory; an actual 5505 (7.2% of invitees) participated. The participation rate decreased from 10.8% to 7.6% to 5.6% in the three consecutive rounds. Among the people who were invited in all three rounds, 18% participated at least once. People who scored too low in a previous round were still as motivated to respond again on a second invitation (36%) as those who scored high enough (25%).
Altogether, 266 persons tested positive for Ct. The positivity rate was 5.1% in the first round, 5.3% in the second round and 4.2% in the third round (see tables 1 and 2). As shown in figure 1B, the positivity rate increased almost linearly with the number of points scored in the risk score questionnaire, both in 2008 and 2009 but not in 2010, when this rise was not seen with scores higher than 9 (numbers are small though: about 200–300 persons scored 10 or more per year).
NNI and NNS
We calculated that the number of invitations needed to detect one case of chlamydia (NNI) was 182 in the first round in South Limburg and increased to 244 in round 2 and 427 in round 3. The NNS for one Ct-positive was 20, 19 and 24 in rounds 1, 2 and 3, respectively (see table 2).
In comparison, the NNI was significantly lower in Rotterdam than in Amsterdam and in Limburg (table 2), independent of other factors (logistic regression, p<0.001). The NNI clearly relates to the higher participation rates in the two cities than in Limburg (table 2, number tested/number invited), where no selection was applied. The NNS was higher in Amsterdam, while values for Rotterdam were quite similar to Limburg. Due to lower participation rates and similar or slightly reduced positivity rates, the NNI increased significantly from round 1 to rounds 2 and 3. Over the three rounds, the NNS remained lower in Rotterdam and Limburg as compared to Amsterdam.
The number of invitations for one positive Ct-case was consistently higher for men than for women (OR 2.4, 95% CI 2.2 to 2.6 (see online supplementary table 1)) and varied by age group: the age group 20–24 years had a lower NNI than 16–19 years (OR 0.87, 95% CI 0.81 to 0.94), while the 25–29 years group had a higher NNI (OR 1.41, 95% CI 1.3 to 1.5). The NNI was higher in low-risk clusters. The NNS for one positive case was also higher for men than for women (OR 1.13, 95% CI 1.05 to 1.21) and increased with age group (OR 1.4 in 20–24 years and 2.5 in 25–29 years compared to 16–19 years). The NNS was higher in medium- and low-risk areas in Amsterdam and Rotterdam, but this trend was not seen in South Limburg, due to filtering out low-risk participants.
South Limburg: effect of applying stricter selection on NNI and NNS
To investigate the effect of a stricter selection by applying a higher cut-off point, we compared the NNI and NNS at a hypothetical selection score of 5+ to 10+ in rounds 1, 2 and 3. With stricter selection, the NNS would decrease gradually and the NNI would increase (figure 2A), while at the same time, an increasing proportion of the Ct-positives would be missed (figure 2B). At a cut-off point above 7 or 8, the NNI starts to increase sharply, especially with low participation rates as in round 3. At given participation rates, selection of participants at a cut-off score of 7 points instead of 5 or 6 points would not increase the number of invitations needed per case substantially, while it would reduce the NNS by about 25%. However, with this stricter selection, 15%–20% of Ct-positives would be missed.
Development of number needed to invite (NNI) and number needed to screen (NNS) (A) and proportion of Ct-positives missed (B) with increasing cut-off point for selection in Chlamydia Screening Implementation, South Limburg, 2008–2010.
Acceptability of the risk score selection among non-selected
Seventy-six of 200 persons who were excluded from participation (38%) responded to the questionnaire on risk score selection, 29 men (33%) and 47 women (42%). Of these respondents, 11 (15%) mentioned that they had not yet been sexually active; hence, they were actually not supposed to fill in the risk score questionnaire. The respondents approved the screening set-up in general (eg, the information was clear, and internet participation was appreciated). More than 30% indicated they had been disappointed not to be able to participate in CSI. Four persons (5%) thought that they might have been at risk for chlamydia; six people (8%) decided to visit a general practitioner or a STI centre for a chlamydia test. Fifty-eight per cent mentioned that they would like to be able to participate in a future screening programme, and 72% would prefer the same procedure.
Discussion
In this paper, we describe the innovative use of a prediction rule for Ct-infections and show that selection through risk score in South Limburg worked well, as it was able to capture a population for screening in which the chance to detect a chlamydia infection was higher than in the general population. The positivity rates in the screening were 4.2%–5.1% per round in South Limburg, similar to or even higher than that in the cities Rotterdam (5.3%) and Amsterdam (3.1%) and also clearly higher than the national prevalence estimate in an earlier pilot Ct, that is, 2.3% among sexually active 15–29-year-olds.8
In the targeted population of South Limburg, more invitations needed to be sent out to detect one Ct-infection than in the cities, Amsterdam and Rotterdam, where participation rates were higher because no selection other than the criterion ‘being sexually active’ was applied. Nevertheless, the NNS was comparable between Limburg and Rotterdam and even higher in Amsterdam. Higher positivity rates in urban areas than in rural areas were expected, based on the pilot study,8 9 but differences between the two cities were also seen, probably relating to different population characteristics (more high-risk clusters in Rotterdam than in Amsterdam) and higher annual testing rates in Amsterdam through a long-established STI clinic (21% of young persons in Amsterdam13 indicated to have been tested for STI in the previous year vs 12% in Rotterdam14). In the third round in Limburg, when the NNI was more than double of that in the first round, the NNS only increased by a fifth, from 20 to 24; hence, the selection remained effective to keep the number of tests to be performed low. The findings show that higher scores were clearly related to higher positivity rates. The fact that the highest scores in round 3 were no longer associated with a higher positivity rate may suggest that CSI had some impact, although others show counter effects in high-risk individuals being re-infected repeatedly after screening and treatment.3
Selection by risk score can improve cost-effectiveness of a screening programme, provided the costs of testing are a considerable part of the total costs. The extrapolation of the findings in our study shows that a stricter selection could be implemented to make the screening more effective, that is, at a score of 6 or 7 rather than 5 points. However, cut-off scores higher than 7 would result in missing too many Ct-positives. Our results, retrospectively, for the cities suggest that selection by risk score can be applied here as well. Furthermore, our findings indicate that the screening might also become more effective (based on a lower NNI), when rolled out in specific geographic areas, that is, restricted to high- and medium-risk neighbourhoods, or limited to specific groups in the population, that is, inviting only women for screening. Women are more likely to participate in chlamydia screening and have a higher chance to test positive, nevertheless preferably men should be targeted for screening as well.15 16
Invitees in South Limburg who were denied the opportunity to be tested because of a low score were frequently disappointed by this. Although they still approved the screening set-up in general, this is a potential drawback of selection and should be addressed by more careful explanation about the selection procedure and the ‘momentary’ value of the risk score. On the other hand, potential participants in subsequent rounds know about the selection by risk score, so we cannot rule out some misclassification by intention when participants ‘lie’ to get higher scores to prevent exclusion.
The risk score we used was developed in an earlier pilot study,9 where it was calculated that, with a minimum selection score of 6, the sensitivity was 93% (proportion of cases detected) by testing 62% of the population (specificity 38%). In our setting, low-risk responders were excluded, so we cannot calculate the sensitivity/specificity, but we tested a similar proportion (64%) of the population. The novelty of this scoring system was the use of a point-score per question, different for male and female respondents, adding up to a total score, to which a cut-off point was applied. Other selective screening approaches investigated in the past used single selective criteria and have booked varying results. In a relatively low-risk population, low diagnostic accuracy was reported,17 leaving a relatively large part of cases undetected. In more high-risk settings, such as family planning and STI clinics, better results were reported.18 19 The criteria used for selection were quite similar, including age, ethnicity, sexual behaviour and symptoms, but the application of a specific score per risk category and a cut-off total score as we used adds to the usefulness of the selection tool in a relatively low-risk population. Within opportunistic screening programmes where recruitment is either via the internet,20 the general practitioner21 or a wider range of healthcare venues,22 a risk score selection will be of great value to standardise the focus on higher risk groups, get more insight in who is applying for a test and make people aware of their own risk level.
Internet-based health interventions facilitate interactive communication with the target population; this has been used in a wide range of interventions, from psychological therapy and weight counselling to sexual health information. It enables tailor-made interventions. Self-scoring of risk factors in order to assess one's own risk has been applied widely on medical information websites (ie, breast cancer, diabetes, depression). The use of risk scores directly linked to entry into an intervention programme has been tried before in gastric carcinoma23 24 and diabetes25; however, it has so far only anecdotally been applied in the context of STI screening. A web-based ‘eTriage’ tool was used for triage and booking appointments in genitourinary medicine clinics in London.26 Risk and symptom self-assessment was used as a way to select male patients visiting a sexual health clinic in the UK,27 and computer-assisted self-interviews for sexual history taking have been shown to be reliable, efficient and highly acceptable in clinical sexual health settings28 29 and general practices30 in Australia. We think such a selection tool in the setting of an online accessible STI testing website can be very helpful to facilitate case detection and treatment within the target group of young people, who tend to use the internet for private medical matters frequently already.
Investigating the possibilities of a selection by risk score in a larger setting of chlamydia screening, either systematic screening (also in the cities, more national coverage) or more opportunistic (eg, in STI centres before appointments for testing are made), is recommended. For implementation in other countries, the specific questions and answers with scores should be adjusted and validated for the target group and area.
Key messages
Systematic screening for chlamydia will reach all persons in the target group but includes those at no/low risk.
Selection of participants by risk profile can be attained by a simple short questionnaire linked to a score.
Selection based on risk score proved highly effective in yielding high positivity rates among the group tested.
We recommend application of a selection tool in a variety of STI testing settings, especially when combined with online communication.
Acknowledgments
The authors gratefully acknowledge Dr M.A.B. van der Sande for comments on earlier versions of the manuscript and G. Doornbos and M. Meijer for assistance in data management.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Download Supplementary Data (PDF) - Manuscript file of format pdf
Footnotes
Funding The Dutch Organisation for Health Research and Development (ZonMw). Project number 12.400.0001. They are the financial administrative office for research for the Ministry of Health, Welfare and Sports; the Chlamydia Screening Implementation Programme is being carried out by request of the Ministry of Health, Welfare and Sport.
Competing interests None.
Patient consent The data analysis has been done with completely anonymised data; the results are only presented as aggregated per group. Patient questionnaires were voluntary.
Ethics approval The ethics committee of the Free University of Amsterdam (METC number: 2007/239) has approved the study, which conforms to national and international legislation.
Provenance and peer review Not commissioned; externally peer reviewed.